Introducing CRISPR-TSKO: A Breakthrough in Precision Gene Editing
An excellent way to explore the function of a gene is to knockout its expression and see what happens. The development of the targeted gene editing system CRISPR (clustered regularly interspaced short palindromic repeats) has triggered scores of gene knockout studies. CRISPR is based on a defense system used by prokaryotes against the nucleic acids of their invaders. The invader’s DNA is cleaved by the DNA nuclease CRISPR-associated 9 (Cas9) and a guide RNA (gRNA) that directs the nuclease to the invader’s DNA. This system has been adapted to cleave DNA sequences of choice in plants using artificially generated gRNAs designed to direct the nuclease to target genes. The double-strand breaks produced by Cas9 are repaired by the error-prone non-homologous end joining pathway, generating short insertions and/or deletions at the break site to knockout target gene function in an inheritable manner (Bortesi and Fischer, 2015).
Sounds easy, but how do you target one of the ~10% of essential genes in a plant whose knockout would have grave consequences and prevent the generation of homozygous mutants for analysis? One approach is to create gene knockouts only in particular tissues or organs using specific promoters to drive Cas9 expression. Decaestecker, Buono et al. (2019) created a comprehensive toolkit to do just that in Arabidopsis thaliana called CRISPR-TSKO (tissue-specific knockout vector system). CRISPR-TSKO is based on the popular Golden Gate technology and GreenGate vectors (Lampropoulos et al., 2013), which were modified to contain selectable and/or non-destructive fluorescent markers to allow for easy screening in the T1 generation.
 First, in transgenic plants already expressing GFP (green fluorescent protein), the authors targeted root cap cells by expressing Cas9 transcriptionally fused to mCherry driven by the root cap-specific SOMBRERO (SMB) promoter and a gRNA targeting GFP. Plants generating mCherry signals tended to lose GFP fluorescence specifically in the root cap, indicating that mCherry-Cas9 successfully targeted GFP only in these cells. Young root cap cells expressed both GFP and mCherry signals, pointing to the need for GFP mRNA and/or protein turnover before the knockout phenotype becomes apparent. When SMB itself was targeted, plants with the smb mutant phenotype were produced, with enlarged root caps and delayed, aberrant root cap cell death. This phenotype was observed in all transgenic T2 seedlings, indicating that the CRISPR-TSKO-induced mutations were strictly somatic and that the mutagenic effect could be inherited.
First, in transgenic plants already expressing GFP (green fluorescent protein), the authors targeted root cap cells by expressing Cas9 transcriptionally fused to mCherry driven by the root cap-specific SOMBRERO (SMB) promoter and a gRNA targeting GFP. Plants generating mCherry signals tended to lose GFP fluorescence specifically in the root cap, indicating that mCherry-Cas9 successfully targeted GFP only in these cells. Young root cap cells expressed both GFP and mCherry signals, pointing to the need for GFP mRNA and/or protein turnover before the knockout phenotype becomes apparent. When SMB itself was targeted, plants with the smb mutant phenotype were produced, with enlarged root caps and delayed, aberrant root cap cell death. This phenotype was observed in all transgenic T2 seedlings, indicating that the CRISPR-TSKO-induced mutations were strictly somatic and that the mutagenic effect could be inherited.
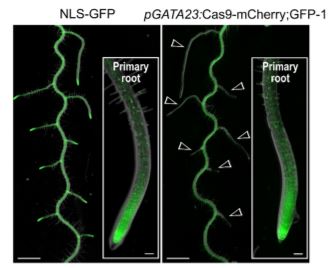
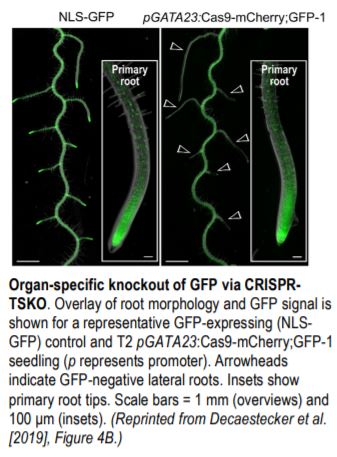
The authors then targeted the mitogen-activated protein kinase gene YODA (YDA), whose mutants have clustered stomata and severely arrested growth. This was accomplished using Cas9-mCherry driven by the stomate-specific TOO MANY MOUTHS promoter and a gRNA targeting YDA. All 40 T1 mCherry-positive seedlings had clustered stomata but no growth arrest. All mutants grown in soil were fertile, whereas homozygous yda mutants cannot be maintained, highlighting the enormous potential of CRISPR-TSKO. The authors also targeted GFP with Cas9-mCherry;GFP driven by the GATA23 promoter, which is expressed in lateral root founder cells. The mCherry-positive T1 seedlings showed little or no GFP expression in lateral roots but normal GFP expression in primary roots (see figure). Therefore, whole organs devoid of the function of a gene of interest can now be generated using CRISPR-TSKO.
In total, Decaestecker, Buono et al. targeted nine genes, including genes essential for plant growth, development, and/or reproduction, using four promoters specific to different tissues or organs. The functions of many more essential genes in particular tissues during specific times in a plant’s lifecycle can now be explored using CRISPR-TSKO.
Jennifer Lockhart, Science Editor
ORCID: 0000-0002-1394-8947
REFERENCES
Bortesi, L., and Fischer, R. (2015). The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnology Advances 33, 41-52.
Decaestecker, W., Buono, R.A., Pfeiffer, M.L., Vangheluwe, N., Jourquin, J., Karimi, M., Van Isterdael, G., Beeckman, T., Nowack, M.K., and Jacobs, T.B. (2019). CRISPR-TSKO: A Technique for Efficient Mutagenesis in Specific Cell Types, Tissues, or Organs in Arabidopsis. Plant Cell 31: https://doi.org/10.1015/tpc.19.00454.
Lampropoulos, A., Sutikovic, Z., Wenzl, C., Maegele, I., Lohmann, J.U., and Forner, J. (2013). GreenGate – A Novel, Versatile, and Efficient Cloning System for Plant Transgenesis. PLOS One 8, 15.