Illuminating photosynthesis in the mesophyll of diverse leaves
Meisha Holloway-Phillips
Department of Environmental Sciences, University of Basel, Switzerland
Since Terashima and Saeki (1983) demonstrated that light attenuation through the leaf was accompanied by spectral changes, there has been increasing interest in how light absorption profiles align with photosynthetic capacity. Progress in our understanding has come from painstaking work relating the internal distribution of chlorophyll content and photosynthetic capacity (rubisco, electron transport rate or 14C fixation) to light absorption and realized photosynthesis through the leaf (e.g., Terashima and Inoue, 1985; Nishio et al., 1993; Vogelmann and Evans, 2002; Evans and Vogelmann, 2003; Evans and Vogelmann, 2006). From these, and investigations by others, four general conclusions have been made:
1) Light absorbed/chlorophyll varies with leaf depth and the pattern is wavelength dependent. Red and blue light are more strongly absorbed by chlorophyll compared with green light, so that their attenuation through the leaf profile is steeper. Thus, to capture weakly absorbing green light at depth, more chlorophyll per photons absorbed is required to drive photosynthesis.
2) Light absorption is well approximated by the Beer-Lambert law, which is intimately linked to the chlorophyll distribution as well as being dependent on the apparent extinction coefficient (e.g., Terashima and Saeki, 1985; Evans, 1995; Evans and Vogelmann, 2003).
3) Leaf anatomy plays an important role in distributing light throughout the leaf profile. For example, the columnar palisade cells have been suggested to act as light guides that direct light deeper into the leaf, along with structures such as the bundle sheath extension, sclereids and cystoliths. By contrast, the spherical shape of spongy mesophyll cells and intercellular airspaces have been shown to increase light scattering and, consequently, the apparent extinction coefficient (see reviews by e.g., Smith et al., 1997).
4) Rubisco/chlorophyll varies with leaf depth. In bifacial leaves such as Spinacia oleracea (spinach), where there are distinct palisade and spongy mesophyll layers, rubisco/chlorophyll declines towards the abaxial surface (Evans and Vogelmann, 2003). By comparison, in isobilateral leaves such as Eucalyptus pauciflora with palisade tissue beneath both surfaces and spongy mesophyll in the central zone, photosynthetic capacity/chlorophyll is similarly high at each leaf surface, reaching a minimum in the central zone of the leaf (Evans and Vogelmann, 2006).
 These observations support the idea that photosynthesis at the whole leaf level will be maximized when light absorption perfectly matches the distribution of photosynthetic capacity (Farquhar et al., 1989). Thus, if you invert horizontally displayed leaves which receive light on the adaxial surface during growth, there will be a mismatch between light absorption and photosynthetic capacity and net photosynthesis will be reduced (e.g., Sun and Nishio, 2001), whereas in vertically displayed leaves which receive light on both sides during growth, photosynthetic responses to light will be similar when either leaf surface is illuminated (e.g. Kirschbaum, 1987; DeLucia et al., 1991).
These observations support the idea that photosynthesis at the whole leaf level will be maximized when light absorption perfectly matches the distribution of photosynthetic capacity (Farquhar et al., 1989). Thus, if you invert horizontally displayed leaves which receive light on the adaxial surface during growth, there will be a mismatch between light absorption and photosynthetic capacity and net photosynthesis will be reduced (e.g., Sun and Nishio, 2001), whereas in vertically displayed leaves which receive light on both sides during growth, photosynthetic responses to light will be similar when either leaf surface is illuminated (e.g. Kirschbaum, 1987; DeLucia et al., 1991).
The implication of these observations is that leaf angle may be coordinated with the internal organization of leaf tissues and the distribution of rubisco and chlorophyll, according to different sunlight environments and stress levels: an idea proffered by Smith et al. (1997). A number of models have been developed to investigate optimal solutions (e.g., Buckley and Farquhar, 2004; Ho et al., 2016); however, empirical data to utilize and test these models are severely lacking.
In this issue of Plant Physiology, Borsuk and Brodersen (2019) provide us with these much needed data, with the largest assessment to date of spatially-resolved chlorophyll distributions across a diverse range of leaf forms. Using epi-illumination fluorescence microscopy to estimate chlorophyll distributions, the authors obtained profiles of fifty-seven species from eight major terrestrial plant clades. Using hierarchical cluster analysis, they observed six groups of species with similar chlorophyll profiles that, except for one species group (Eichhornia crassipes), could be described according to a quadratic regression with respect to relative leaf depth. Interestingly, the largest groups tended to be phylogenetically diverse, and anatomical traits anticipated to influence chlorophyll distribution—including leaf thickness, palisade fraction and vein depth—did not consistently explain differences between the clusters.
When the chlorophyll distributions were averaged at the clade level there was also a weak relationship between the depth of peak chlorophyll content and anatomical traits. However, leaf absorption profiles performed on a subset of five species indicated that light absorption, particularly for green light, overlapped within a relatively narrow range of mesophyll coincident with the positioning of the palisade to spongy transition and location of the veins. The authors suggested that this may represent an optimum in tissue organization that ensures absorption of weaker light is maximized from either direction of illumination, and strengthens the growing understanding of the role of green light in driving photosynthesis within deep leaf tissue (Evans, 1995; Terashima et al., 2009).
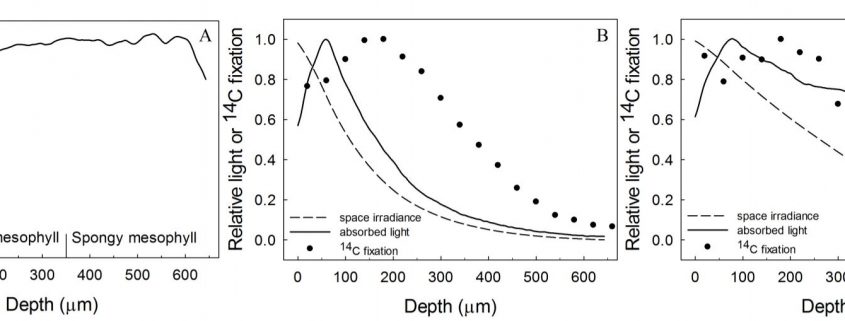
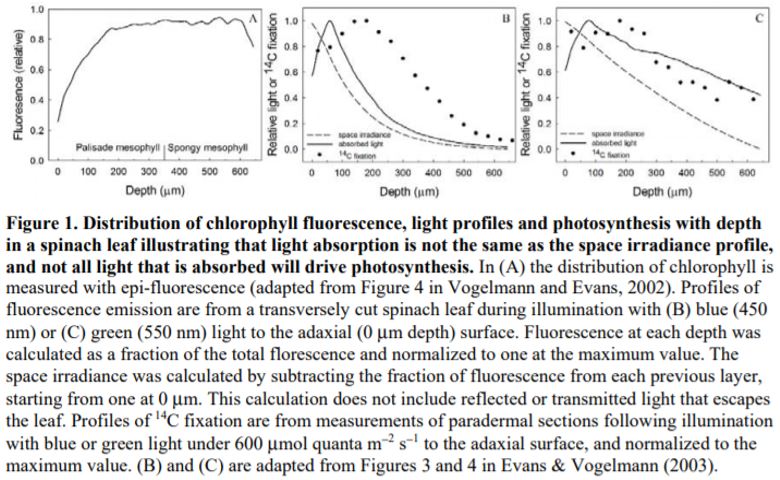
Flexibility in leaf functional design was evident from the individual species profiles, with examples of bimodal (e.g., Zea mays, Bowenia spectabilis), abaxially-weighted (e.g., Araucaria araucana, Ficus subcordata) and near-constant (e.g., Aristolochia gigantea, Anacardium occidentale) chlorophyll distributions. Thus, even though a universal model to predict chlorophyll distribution performed well, I would argue it is in understanding this diversity where we are likely to elucidate the important role leaf internal structure plays in resource allocation and photosynthesis. This is because of a number of depth-specific factors that influence the translation of chlorophyll profiles to photosynthesis (see figure), including a gradient in chloroplast properties analogous to the comparisons found between “sun” and “shade” leaves (e.g., Terashima and Inoue, 1985), and in the organization of the major cell types (i.e., epidermis, palisade and spongy mesophyll) that have been shown to influence the capture and internal processing of absorbed light (e.g., Vogelmann, 1993). These functional relationships can be further modified by acclimation responses to abiotic stress (e.g., chloroplast movement: Inoue and Shibata, 1974; leaf inclination: Ludlow and Björkman, 1984; biochemistry: Chow and Anderson, 1987). Importantly, these factors may be related to competing biophysical demands placed on leaves, including for mechanical support, diffusion of CO2 to the sites of carboxylation and liquid water transport to the sites of evaporation, which similarly depend on leaf structure (Smith et al., 1997). To test the relevance of these factors to the functional interpretation of chlorophyll distributions, there is a similar need for spatially resolved photosynthesis profiles. Historically, this has been a laborious process, but new approaches such as the novel multicolor laser light sheet microscopy technique of Lichtenberg et al. (2017) are a promising way forward.
The rich diversity identified in the work of Borsuk and Broderson (2019) is an important contribution by which to test how well optimized to the prevailing light conditions the distribution of chlorophyll is through a leaf, and supports growing recognition of the need to consider the internal workings of the leaf to improve the reliability of leaf scale models.
Acknowledgements
My thanks to John Evans for providing me with the data from Evans & Vogelmann (2003), and to Graham Farquhar for providing comments on the initial version.