Hydrogen Cyanide Regulation by S-Cyanylation
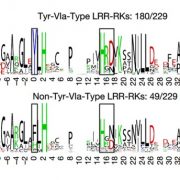
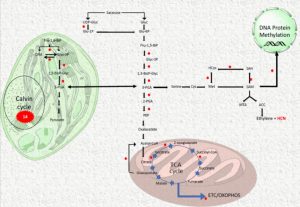 Despite its toxicity, cyanide has been proposed to act as a regulator of several biological processes such as seed dormancy and germination, resistance to fungal and viral infection. Arabidopsis null mutants of the mitochondrial enzyme β-CYANOALANINE SYNTHASE (CAS-C1) accumulate cyanide to apparently nontoxic levels, as the plants are completely viable, but show a root hairless phenotype suggesting a signaling role in root development. Garcia et al. (10.1104/pp.18.01083) have performed a quantitative proteomic analysis of mitochondrially enriched samples isolated from cas-c1 mutants and wild type. Their analysis reveals significant changes in protein content: 451 proteins were absent or less abundant in cas-c1 and 353 proteins were only present or more abundant in cas-c1. Gene ontology classification of these proteins identified proteomic changes that explain the root hairless phenotype and the altered immune response observed in the cas-c1 mutant. The authors analyzed the molecular mechanism by which cyanide can act as a signaling molecule. Gene Ontology classification and protein-protein interaction cluster analysis showed that S-cyanylation is involved in the regulation of primary metabolic pathways, such as glycolysis, and the Calvin and S-adenosyl-Met cycles.
Despite its toxicity, cyanide has been proposed to act as a regulator of several biological processes such as seed dormancy and germination, resistance to fungal and viral infection. Arabidopsis null mutants of the mitochondrial enzyme β-CYANOALANINE SYNTHASE (CAS-C1) accumulate cyanide to apparently nontoxic levels, as the plants are completely viable, but show a root hairless phenotype suggesting a signaling role in root development. Garcia et al. (10.1104/pp.18.01083) have performed a quantitative proteomic analysis of mitochondrially enriched samples isolated from cas-c1 mutants and wild type. Their analysis reveals significant changes in protein content: 451 proteins were absent or less abundant in cas-c1 and 353 proteins were only present or more abundant in cas-c1. Gene ontology classification of these proteins identified proteomic changes that explain the root hairless phenotype and the altered immune response observed in the cas-c1 mutant. The authors analyzed the molecular mechanism by which cyanide can act as a signaling molecule. Gene Ontology classification and protein-protein interaction cluster analysis showed that S-cyanylation is involved in the regulation of primary metabolic pathways, such as glycolysis, and the Calvin and S-adenosyl-Met cycles.