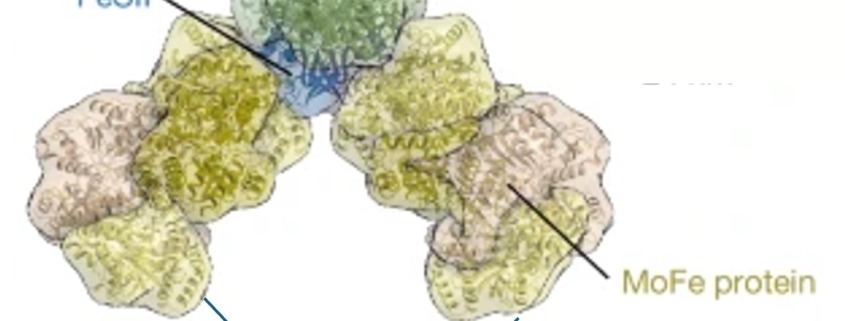
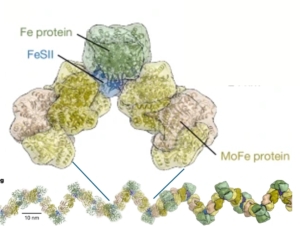
How nitrogenase stays active
One of the great dilemmas of science is the fact that nitrogen gas, though very abundant in the atmosphere, is limiting for most forms of life. Of course, this lack of availability is because N2 gas has an extremely strong triple bond holding the two nitrogen atoms together; it’s so strong that N2 gas is considered “inert” and foods are often packaged in N2 to prolong shelf life. Fortunately, some prokaryotes produce an enzyme, nitrogenase, that can break this triple bond to produce ammonium (NH4+), which can be taken up and used by other organisms. However, this essential enzyme has an Achilles heel, which is that it is rapidly inactivated by oxygen. Fortunately, many nitrogen-fixing organisms produce a protein called FeSII (also known as Shethna protein II) that protects nitrogenase from oxygen, in a mechanism that has just been revealed in two back-to-back articles by Franke et al. and Narehood et al. Nitrogenase is made up of two protein complexes [iron protein (FeP) and molybdenum–iron protein (MoFeP)] containing several metal cofactors. The authors used cryo-EM to image this complex in the presence and absence of oxygen, and they found that in the presence of oxygen, FeSII reversibly binds to these metal-containing regions, protecting them from oxidative damage. Interestingly, the authors also found that the single protected structures can multimerize further into higher-order filaments. Not only is this beautiful work, but it also provides key insights that can one day be used to engineer plants that can fix nitrogen on their own. (Summary by Mary Williams @PlantTeaching.bksy.social @PlantTeaching) Nature https://doi.org/10.1038/s41586-024-08355-3 and https://doi.org/10.1038/s41586-024-08311-1.