Heat shock proteins support refolding and shredding of misfolded proteins
Author: Masanori Izumi
Affiliation: RIKEN Center for Sustainable Resource Science, Wako 351-0198, Japan
Newly synthesized proteins must be folded to form their proper 3D structures. Stresses perturb protein folding, thereby leading to the hyperaccumulation of misfolded or aggregated proteins. Such disrupted proteostasis causes proteotoxic stress that impairs cellular functions, and organisms must refold or remove misfolded proteins to maintain healthy growth. Molecular chaperones that disaggregate misfolded proteins and the cytoplasmic 26S proteasome, which shreds ubiquitinated proteins, are the predominant players in the response to misfolded protein accumulation in eukaryotes (Saibil, 2013; Amm et al., 2014).
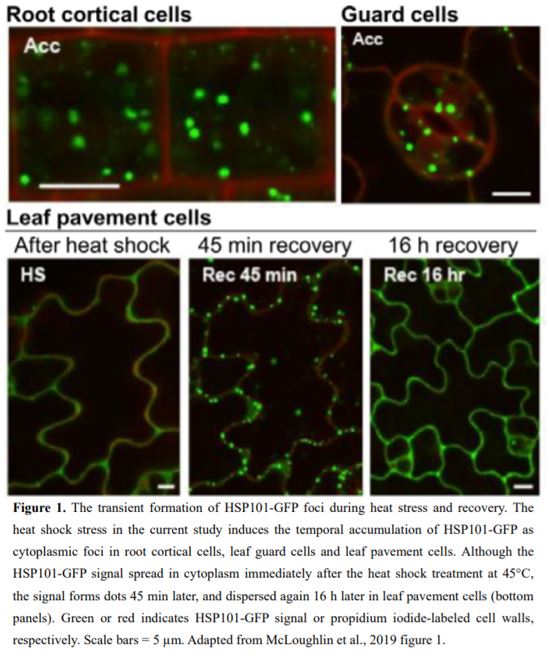 In this issue of Plant Physiology, to further define the roles of the molecular chaperone protein HEAT SHOCK PROTEIN 101 (HSP101), McLoughlin et al. (2019) assessed the intracellular localization of green fluorescent protein (GFP)-labeled HSP101 during the adaptation of Arabidopsis thaliana plants to a heat stress. The authors used an experimental system consisting of two sequential high temperature treatments. The first treatment is a 1.5-h incubation at 38°C as an acclimation to heat stress. Following 2 h of growth at 22℃, plants are subjected to second high temperature treatment at 45°C for 1 h as a heat shock treatment. Then, the treated plants are grown at 22°C again as a recovery period. The authors found that HSP101-GFP transiently appears as small, cytoplasmic dots in various tissues during the heat stress treatment (Fig. 1).
In this issue of Plant Physiology, to further define the roles of the molecular chaperone protein HEAT SHOCK PROTEIN 101 (HSP101), McLoughlin et al. (2019) assessed the intracellular localization of green fluorescent protein (GFP)-labeled HSP101 during the adaptation of Arabidopsis thaliana plants to a heat stress. The authors used an experimental system consisting of two sequential high temperature treatments. The first treatment is a 1.5-h incubation at 38°C as an acclimation to heat stress. Following 2 h of growth at 22℃, plants are subjected to second high temperature treatment at 45°C for 1 h as a heat shock treatment. Then, the treated plants are grown at 22°C again as a recovery period. The authors found that HSP101-GFP transiently appears as small, cytoplasmic dots in various tissues during the heat stress treatment (Fig. 1).
Previous studies revealed that heat stress induces the formation of a type of membrane-less organelle termed a stress granule to alleviate proteotoxic stress (Cherkasov et al., 2013; Mitrea and Kriwacki, 2016). HSP101-GFP-labeled foci, which appeared to move gradually, were temporally associated with stress granules labeled by a stress granule marker POLY(A) BINDING PROTEIN 2 (PAB2)-red fluorescent protein (RFP) fusion (Sorenson and Bailey-Serres, 2014). The heat stress-induced HSP101 foci also showed transient colocalization with the puncta that were visualized by a regulatory subunit of 26S proteasome RPN1a-RFP fusion. The biochemical identification of HSP101-interacting proteins also suggested the interaction between HSP101 and 26S proteasome subunits including RPN1a. In the hsp101 mutant plants, the increase in insoluble or ubiquitinated proteins after heat shock is enhanced compared with wild-type plants. These findings indicate a cooperative role of HSP101 with the 26S proteasome to degrade ubiquitinated proteins produced by heat shock stress.
The comparison of protein status in soluble and insoluble fractions between wild-type and hsp101 plants through MS spectrometry supported the conclusion that HSP101 disaggregates numerous proteins to help refolding or proteasomal shredding. Overall, the current study points to an important role of HSP proteins in the coordination between stress granules and the 26S proteasome system to overcome impaired proteostasis due to heat stress. An interesting question is how and why HSP101 transiently forms cytoplasmic foci in response to heat stress. Are there large protein complexes containing the components of stress granules or proteasomes? Are aggregated proteins also concentrated there? The mechanism and physiological importance of the transient formation of HSP101-containing foci remains uncertain. Further characterization of plant adaptive strategies for proteotoxic stress would allow researchers to establish effective approaches to generate stress-tolerant crops.
Literature cited
Amm I, Sommer T, Wolf DH (2014) Protein quality control and elimination of protein waste: The role of the ubiquitin-proteasome system. Biochim Biophys Acta 1843: 182-196
Cherkasov V, Hofmann S, Druffel-Augustin S, Mogk A, Tyedmers J, Stoecklin G, Bukau B (2013) Coordination of translational control and protein homeostasis during severe heat stress. Curr Biol 23: 2452-2462
Fionn McLoughlin F, Kim M, Marshall RS, Vierstra RD Vierling E (2019) Hsp101 interacts with the proteasome and promotes the clearance of ubiquitylated protein aggregates. Plant Physiol
Mitrea DM, Kriwacki RW (2016) Phase separation in biology; functional organization of a higher order. Cell Commun Signal 14: 1
Saibil H (2013) Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Bio 14: 630-642
Sorenson R, Bailey-Serres J (2014) Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc Natl Acad Sci U S A 111: 2373-2378