Flexibility of intrinsically disordered degrons in AUX/IAA proteins reinforces auxin receptor assemblies ($) (BioRxiv)
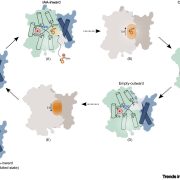
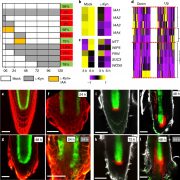
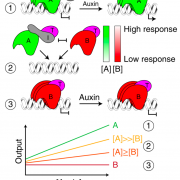
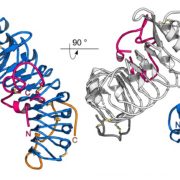
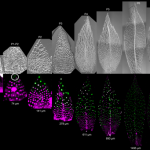
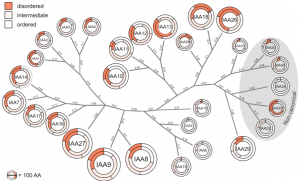 Auxin is involved in multiple plant development and stress response which necessitates complexity in auxin signaling. Auxin at a particular threshold in the cells brings together the TIR1/AFB1-5 (TRANSPORT INHIBITOR RESPONSE1/ AUXIN SIGNALING F-BOX 1-5) members and members of the 29 Aux/IAA members bound to the activator ARF (AUXIN RESPONSE FACTOR) transcriptional factors. This complex is presented for ubiquitin-mediated degradation by the SCF complex which alters Aux/IAA levels and repression state of the ARF proteins. In this paper, the authors investigated the complexity in structural properties of two Aux/IAA proteins, IAA7 and IAA12 which can alter the TIR1-Aux/IAA complex. IAA7 and IAA12 possess an intrinsically disordered domain that changes the protein conformation and plays a vital role in the auxin signaling through specific interaction with TIR1. This structural information about the Aux/IAA –AUXIN-TIR1 interaction complex will aid in understanding the regulation of auxin-responsive gene expression necessary for plant growth. Summary by Suresh Damodaran. BioRxiv, DOI : http://dx.doi.org/10.1101/787770.
Auxin is involved in multiple plant development and stress response which necessitates complexity in auxin signaling. Auxin at a particular threshold in the cells brings together the TIR1/AFB1-5 (TRANSPORT INHIBITOR RESPONSE1/ AUXIN SIGNALING F-BOX 1-5) members and members of the 29 Aux/IAA members bound to the activator ARF (AUXIN RESPONSE FACTOR) transcriptional factors. This complex is presented for ubiquitin-mediated degradation by the SCF complex which alters Aux/IAA levels and repression state of the ARF proteins. In this paper, the authors investigated the complexity in structural properties of two Aux/IAA proteins, IAA7 and IAA12 which can alter the TIR1-Aux/IAA complex. IAA7 and IAA12 possess an intrinsically disordered domain that changes the protein conformation and plays a vital role in the auxin signaling through specific interaction with TIR1. This structural information about the Aux/IAA –AUXIN-TIR1 interaction complex will aid in understanding the regulation of auxin-responsive gene expression necessary for plant growth. Summary by Suresh Damodaran. BioRxiv, DOI : http://dx.doi.org/10.1101/787770.