Every pair is special!
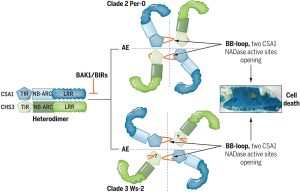
The identification and characterization of resistosome complexes, formed by the oligomerization of intracellular nucleotide-binding leucine-rich repeat receptors (NLRs), has recently been a major focus in the field of plant immunity. Some NLR genes occur as head-to-head pairs encoding proteins that function as heterodimers. Yang et al. investigated the oligomerization requirements for the paired class of NLRs using the Arabidopsis CHS3-CSA1 pair. Using blue native-PAGE and co-IPs, the authors show that clades 2 and 3 of CHS3-CSA1 form heterodimer structures. They further show that upon activation (for example upon effector binding), these heterodimers further oligomerize to form heterotetrameric complexes. Through a series of mutational experiments, the authors further identify specific domain features required for the function and oligomerization of these complexes. Finally, building upon previous studies linking CHS3-CSA1 to the immune co-receptors BAK1 and/or BIRs, the group shows that these co-receptors act as negative regulators of CHS3-CSA1 and inhibit their further oligomerization to the heterotetrameric state. The authors close by highlighting how the requirements they identified for the function and oligomerizing of CHS3-CSA1 differ significantly from another well-studied NLR pair, RRS1-RPS4. These findings serve as an important reminder that overgeneralizations and predictions based off limited representative structures may not capture significant differences in paired NLR structure. (Summary by Tamar Av-Shalom @TamarAvShalom) Science. 10.1126/science.adk3468