Designer pentatricopeptide repeat PPR protein as RNA affinity tag (Plant Cell)
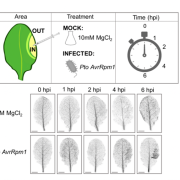
 Pentatricopeptide repeat (PPR) proteins bind RNA, using a code in which specific amino acid motifs recognize specific bases, similar to TAL effector proteins. In plants, PPR proteins localize almost exclusively to mitochondria and chloroplasts, with functions including RNA, stabilization, splicing and editing. Here, McDermott et al. used insights about the amino-acid to RNA code to design artificial PPR (aPPRs) proteins that bind target RNAs with high specificity in vivo. These aPPRs can then be used to isolate specific ribonucleoprotein particles (RNPs; associations of RNA + proteins) to characterize their constituents. The authors designed an aPPR that specifically binds the 3’ UTR of the chloroplast psbA mRNA, which encodes photosystem II protein D1. Their study identified psbA mRNA binding proteins of unknown function, and also demonstrated the feasibility of using aPPRs to characterize proteins that bind to specific RNAs. (Summary by Mary Williams) Plant Cell 10.1105/tpc.19.00177
Pentatricopeptide repeat (PPR) proteins bind RNA, using a code in which specific amino acid motifs recognize specific bases, similar to TAL effector proteins. In plants, PPR proteins localize almost exclusively to mitochondria and chloroplasts, with functions including RNA, stabilization, splicing and editing. Here, McDermott et al. used insights about the amino-acid to RNA code to design artificial PPR (aPPRs) proteins that bind target RNAs with high specificity in vivo. These aPPRs can then be used to isolate specific ribonucleoprotein particles (RNPs; associations of RNA + proteins) to characterize their constituents. The authors designed an aPPR that specifically binds the 3’ UTR of the chloroplast psbA mRNA, which encodes photosystem II protein D1. Their study identified psbA mRNA binding proteins of unknown function, and also demonstrated the feasibility of using aPPRs to characterize proteins that bind to specific RNAs. (Summary by Mary Williams) Plant Cell 10.1105/tpc.19.00177