Crystal structure and pH dependency of peptidase & ligase activity in Arabidopsis legumain (Plant Cell)
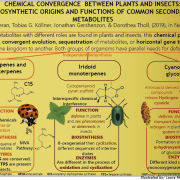
 Legumains are proteins identified first from legumes that have subsequently been identified in other organisms. They are also described as asparaginyl endopeptidases (AEPs) and vacuolar cysteine proteases or vacuolar processing enzymes. Enzymatically, legumains can express peptidase and peptide ligase activities, and have been demonstrated to contribute to the formation of cyclic peptides. Because of the myriad potential applications for cyclic peptides as bioactive agents and pharmaceutical platforms, there is considerable interest in developing engineered, tunable enzymes for their production. Zauner et al. have identified the crystal structure of an Arabidopsis vegetative-type legumain, AtLEGγ, and show that its activity and specificity is strongly pH sensitive, supporting its putative functions in various compartments of the plant cell, but also providing an opportunity to control its activity in vitro. (Summary by Mary Williams) Plant Cell 10.1105/tpc.17.00963
Legumains are proteins identified first from legumes that have subsequently been identified in other organisms. They are also described as asparaginyl endopeptidases (AEPs) and vacuolar cysteine proteases or vacuolar processing enzymes. Enzymatically, legumains can express peptidase and peptide ligase activities, and have been demonstrated to contribute to the formation of cyclic peptides. Because of the myriad potential applications for cyclic peptides as bioactive agents and pharmaceutical platforms, there is considerable interest in developing engineered, tunable enzymes for their production. Zauner et al. have identified the crystal structure of an Arabidopsis vegetative-type legumain, AtLEGγ, and show that its activity and specificity is strongly pH sensitive, supporting its putative functions in various compartments of the plant cell, but also providing an opportunity to control its activity in vitro. (Summary by Mary Williams) Plant Cell 10.1105/tpc.17.00963