Cold-Induced Proteomic Changes in the Chloroplast Envelope
As a cold-hardy species, Arabidopsis (Arabidopsis thaliana), is a useful model for the investigation of the molecular and physiological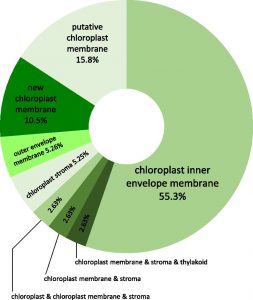 mechanisms underlying cold acclimation in plants. Chloroplasts harbor the enzymatic machinery required for photosynthetic CO2 fixation, starch production, nitrite and sulfate reduction, and amino acid and fatty acid biosynthesis. To fulfill all these functions, chloroplasts must import and export a wide variety of metabolic intermediates, and they have to communicate with the nucleus to balance plastidic and nuclear gene expression. Accordingly, changes in external parameters, such as temperature, necessitate substantial genetic and metabolic readjustments. These considerations led Trentmann et al. (doi.org/10.1104/pp.19.00947) to perform a comparative analysis of chloroplast envelope proteins from cold-treated plants and plants constantly grown under standard conditions. A label-free quantitative proteomic technique was employed because the the specimens used in such studies can be grown on soil and thus under more natural conditions. In total, the authors identified 38 envelope membrane intrinsic or associated proteins exhibiting altered abundance after cold acclimation. Among these proteins are many solute carriers, such as the ATP/ADP antiporter nucleotide transporter2 (NTT2), which increases in abundance during cold acclimation, and maltose exporter1 (MEX1) which decreases in abundance. Analysis of the frost recovery of ntt2 loss-of-function and mex1 overexpressor mutants confirmed that the comparative proteome is well suited to identify key factors involved in cold acclimation and acquisition of freezing tolerance.
mechanisms underlying cold acclimation in plants. Chloroplasts harbor the enzymatic machinery required for photosynthetic CO2 fixation, starch production, nitrite and sulfate reduction, and amino acid and fatty acid biosynthesis. To fulfill all these functions, chloroplasts must import and export a wide variety of metabolic intermediates, and they have to communicate with the nucleus to balance plastidic and nuclear gene expression. Accordingly, changes in external parameters, such as temperature, necessitate substantial genetic and metabolic readjustments. These considerations led Trentmann et al. (doi.org/10.1104/pp.19.00947) to perform a comparative analysis of chloroplast envelope proteins from cold-treated plants and plants constantly grown under standard conditions. A label-free quantitative proteomic technique was employed because the the specimens used in such studies can be grown on soil and thus under more natural conditions. In total, the authors identified 38 envelope membrane intrinsic or associated proteins exhibiting altered abundance after cold acclimation. Among these proteins are many solute carriers, such as the ATP/ADP antiporter nucleotide transporter2 (NTT2), which increases in abundance during cold acclimation, and maltose exporter1 (MEX1) which decreases in abundance. Analysis of the frost recovery of ntt2 loss-of-function and mex1 overexpressor mutants confirmed that the comparative proteome is well suited to identify key factors involved in cold acclimation and acquisition of freezing tolerance.



