Close Encounters of the ARF Kind: Proximity-based ARF1 GTPase Activity Regulates Vesicle Trafficking
ADP-RIBOSYLATION FACTOR (ARF) proteins play essential roles in vesicle trafficking by regulating the formation of membrane vesicles that move cargo throughout the cell. Their activity is controlled by specific guanine exchange factors (ARF-GEFs) that activate ARFs by catalyzing a GDP to GTP exchange that ultimately recruits vesicle coat proteins, and by ARF-GAPs (GTPase ACTIVATING PROTEINs) that hydrolyze GTP back to GDP for protein inactivation (Singh and Jürgens, 2018).
In vitro studies indicate that ARF1-GTPase dimerization is critical for the formation of membrane vesicles in humans (Beck et al., 2011). Moreover, in vivo studies in Arabidopsis demonstrate that ARF-GEFs occur as dimers despite the fact that the ARF-GEF catalytic domain alone is sufficient for in vitro exchange assays (Anders et al., 2008). To better understand the mode of ARF1 activation in vivo, Brumm et al. (2020) explored the dynamics and biological significance of ARF-related protein-protein interactions in Arabidopsis, focusing specifically on the ARF-GEF GNOM involved in endosome-to-plasma membrane trafficking.
 The authors first surveyed protein complex formation by co-immunoprecipitation (co-IP) using wild-type GNOM, endogenous ARF1, and variants of ARF1 that are locked into GDP (T31N) or GTP (Q71L) bound states. Both ARF1-T31N and ARF1-Q71L variants interacted with endogenous ARF1 whereas wild-type ARF1 did not. In addition, strong interactions between GNOM and GDP-locked ARF1-T31N were observed compared to the relatively weak interactions between GNOM and endogenous ARF1 or ARF1-Q71L. Cell fractionation analyses followed by co-IP demonstrated that GNOM forms homodimers in the cytosol and at the membrane, while interactions between GNOM and endogenous ARF1 were detected only at the membrane upon stabilization with the trafficking inhibitor Brefeldin A (BFA). Together these experiments demonstrate that critical steps of GNOM-dependent ARF activation occur at the membrane.
The authors first surveyed protein complex formation by co-immunoprecipitation (co-IP) using wild-type GNOM, endogenous ARF1, and variants of ARF1 that are locked into GDP (T31N) or GTP (Q71L) bound states. Both ARF1-T31N and ARF1-Q71L variants interacted with endogenous ARF1 whereas wild-type ARF1 did not. In addition, strong interactions between GNOM and GDP-locked ARF1-T31N were observed compared to the relatively weak interactions between GNOM and endogenous ARF1 or ARF1-Q71L. Cell fractionation analyses followed by co-IP demonstrated that GNOM forms homodimers in the cytosol and at the membrane, while interactions between GNOM and endogenous ARF1 were detected only at the membrane upon stabilization with the trafficking inhibitor Brefeldin A (BFA). Together these experiments demonstrate that critical steps of GNOM-dependent ARF activation occur at the membrane.
To further assess how ARF1 is activated at the membrane, the authors generated an Arabidopsis ARF1-Y35A variant that was predicted to be dimerization-deficient based on work in mammalian systems (Beck et al., 2011). Fluorescence-based proximity assays confirmed that ARF1 proteins are indeed in close proximity to each other (<10 nm) at the membrane and revealed that the ARF1 Y35A mutation disrupted this process. Functional trafficking assays further demonstrated that the ARF1-Y35A variant could not rescue secretion defects in cells over-expressing inactive ARF1. Indeed, proper vesicle formation and trafficking defects were observed when overexpressing ARF1-Y35A by itself, underscoring the importance of bringing ARF1 monomers into close contact.
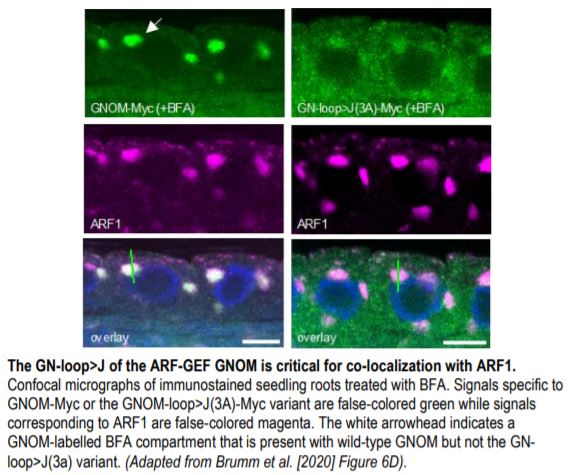
Lastly, the authors reasoned that ARF1 proximity in the membrane was likely established by ARF-GEF dimers. To address this, they investigated the importance of the C-terminal loop of the ARF-GEF catalytic domain (J-loop) shown to be important for binding ARF1 in human cells. Consistent with their hypothesis, a J loop mutant variant of Arabidopsis GNOM (GN-loop>J(3A)) failed to rescue several gnom lines. Moreover, the J-loop variant did not co-localize with ARF1 in Arabidopsis cells treated with BFA (see Figure) and showed a weak interaction with ARF1 in co-IP assays, confirming that functional GNOM dimers are required for ARF1 activation. Collectively, these results highlight the critical nature of active ARF1 spacing and brings forth a new model of ARF1 regulation that relies on ARF-GEF dimerization for their activation and proximity-based functionality.
Philip Carella
Sainsbury Laboratory
University of Cambridge, Cambridge
ORCID: 0000-0002-5467-7290
REFERENCES
Anders N, Nielsen M, Keicher J, Stierhof Y-D, Furutani M, Tasaka M, Skriver K and Jürgens G (2008). Membrane Association of the Arabidopsis ARF Exchange Factor GNOM Involves Interaction of Conserved Domains. Plant Cell 20: 142–151.
Beck R, Prinz S, Diestelkötter-Bachert P, Röhling S, Adolf F, Hoehner K, Welsch S, Ronchi P, Brügger B, Briggs JAG and Wieland F (2011). Coatomer and dimeric ADP ribosylation factor 1 promote distinct steps in membrane scission. The Journal of Cell Biology 194: 765–777.
Brumm S, Singh MK, Nielsen ME, Richter S, Beckmann H, Stierhof Y-D, Fischer A-M, Kumaran M, Sundaresan V and Jürgens G (2020). Coordinated activation of ARF1 GTPases by ARF-GEF GNOM dimers is essential for vesicle trafficking in Arabidopsis. Plant Cell Published June 2020 DOI: https://doi.org/10.1105/tpc.20.00240
Singh MK and Jürgens G (2018). Specificity of plant membrane trafficking – ARFs, regulators and coat proteins. Seminars in Cell & Developmental Biology 80: 85–93.