Cellulose synthase-like D (CSLD) 3 protein is a beta-1,4-glucan synthase
Yang et al. probe the biosynthetic activity of a protein involved in cell wall deposition.
Plant Cell https://doi.org/10.1105/tpc.19.00637
Jiyuan Yang, Erik Nielsen. Department of Molecular, Cellular, and Developmental Biology, University of Michigan, Ann Arbor, MI 48109, USA
Background: As the most abundant organic compound in the world, cellulose microfibrils provide strength and rigidity to the primary cell walls of land plants. It is generally accepted that cellulose is synthesized by large, multi-subunit, membrane-localized cellulose synthase complexes (CSCs), which contain at least three different isoforms of cellulose synthase (CESA) proteins. Each of these CESA proteins synthesizes individual beta-1,4-glucan polysaccharides, which then assemble into cellulose microfibrils. Recent studies have highlighted novel roles for a related class of cellulose synthase-like D (CSLD) proteins in certain types of polarized cell wall deposition. While several different biosynthetic activities have been proposed for CSLD proteins, the nature of the polysaccharides generated by CSLD proteins has remained controversial.
Question: We wanted to definitively establish the biosynthetic activity of one member of the CSLD protein family, CSLD3. To do this, we utilized a combination of biochemical purification and in vitro reconstitution, and genetic complementation methods.
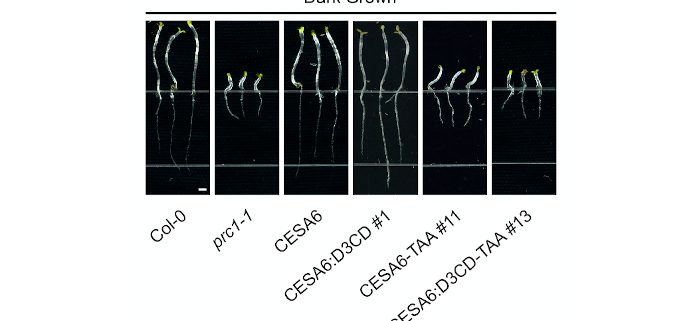
Findings: We found that a chimeric fusion of a cellulose synthase component, CESA6, that contained the catalytic domain of CSLD3, genetically complemented both developmental and cell wall defects in cesa6 mutant plants. These chimeric proteins assembled into functional cellulose synthase complexes, and the catalytic activity of the chimeric fusion proteins was required for these complexes to functionally rescue cesa6 mutant cell wall defects. Furthermore, we demonstrated UDP-glucose dependent β-1,4-glucan synthase activities for purified CESA6 and CSLD3 proteins in vitro, and found that detergent-solubilized CESA6 and CSLD3 proteins assembled into complexes that looked similar to one another, and which displayed some structural similarities to subunits of plant cellulose synthase complexes observed using EM techniques in vivo.
Next steps: It is unclear whether all members of the CSLD protein family are beta-1,4-glucan synthases like CSLD3. In addition, while CESA6 and CSLD3 proteins assembled into similar sized complexes, in planta, these CESA protein complexes further assemble into higher-order cellulose synthase complexes. Whether this occurs for CSLD proteins as well is still unknown, as is the question of whether CSLD-synthesized beta-1,4-glycan polymers assemble into cellulose microfibrils, or some other form of beta-1,4-glucan cell wall polymer.
Jiyuan Yang, Gwangbae Bak, Tucker Burgin, William J. Barnes, Heather B. Mayes, Maria J. Peña, Breeanna Urbanowicz, and Erik Nielsen. (2020). Biochemical and Genetic Analysis Identify CSLD3 as a beta-1,4-glucan Synthase that Functions during Plant Cell Wall Synthesis. Plant Cell; DOI: https://doi.org/10.1105/tpc.19.00637