Autophagy regulation by HLS1-mediated acetylation
Huang et al. explore the molecular mechanism underlying the regulation of autophagy by HLS1.
https://doi.org/10.1093/plcell/koad252
By Li Huang, Xing Wen, Lian Jin, Huihui Han, and Hongwei Guo
New Cornerstone Science Laboratory, Institute of Plant and Food Science, Department of Biology, School of Life Sciences, Southern University of Science and Technology (SUSTech); Shenzhen, Guangdong, 518055, China.
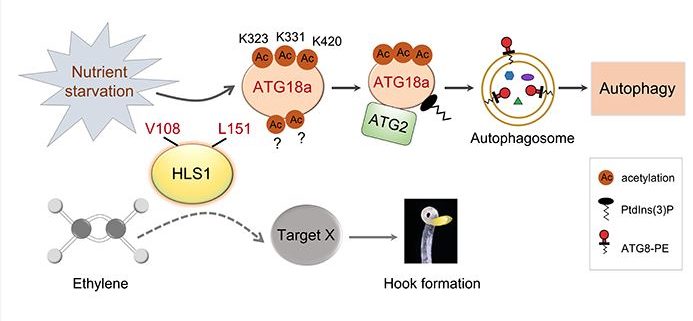
Background: Autophagy is a highly conserved process that delivers cytoplasmic components to the vacuole or lysosome for breakdown and recycling during stresses such as nutrient starvation. Almost every pivotal process of autophagy in yeast and mammals is regulated by an important post-translational modification (PTM) called protein acetylation. Nevertheless, whether and how core autophagy proteins in plants are regulated by acetylation remains elusive. HOOKLESS1 (HLS1) is a putative N-acetyltransferase, but its biochemical function has remained largely unclear. The loss-of-function mutant hls1-1 displays similar phenotypes to autophagy-defective (atg) mutants in senescence and immune responses, suggesting that there may be a relationship between HLS1 and plant autophagy.
Question: Is HLS1 involved in plant autophagy? Does HLS1 regulate autophagy? How does HLS1 function in autophagy?
Findings: We mainly uncovered the following four findings: (1) HLS1 is crucial for triggering autophagy during nutrient starvation in Arabidopsis (Arabidopsis thaliana); (2) HLS1 is a bona fide lysine acetyltransferase that can directly physically interact with and acetylate a key autophagy-related protein (ATG18a) in response to nutrient starvation; (3) HLS1-mediated ATG18a acetylation affects the ATG2-ATG18a interaction and the binding of ATG18a to phosphatidylinositol 3-phosphate to promote autophagy activation and plant responses to nutrient deprivation; and (4) the normal enzymatic activity of HLS1 is also important for apical hook development of etiolated seedlings, but HLS1-regulated autophagy by acetylating ATG18a is uncoupled from HLS1-mediated hook formation.
Next steps: We aim to explore how nutrient starvation modulates HLS1-mediated autophagy via ATG18a acetylation. Moreover, additional HLS1 substrates in multiple biological processes such as hook development are worthy of further investigation.
Reference:
Li Huang, Xing Wen, Lian Jin, Huihui Han, and Hongwei Guo. (2023). HOOKLESS1 acetylates AUTOPHAGY-RELATED PROTEIN18a to promote autophagy during nutrient starvation in Arabidopsis. https://doi.org/10.1093/plcell/koad252