Attacking the front and the rear – the elaborate control of reactive oxygen species production
Kimura et al. reveal how phosphorylation of a key enzyme activates reactive oxygen species production. Plant Cell https://doi.org/10.1105/tpc.19.00525
Sachie Kimuraa,b, Michael Wrzaczeka
aOrganismal and Evolutionary Biology Research Programme, Viikki Plant Science Centre, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, FI-00014, Finland.
bPresent address: Ritsumeikan Global Innovation Research Organization, Ritsumeikan University, 525-8577, Shiga, Japan.
Background: In order to thrive in an ever-changing environment, plants use proteins, which localize to the plasma membrane of the cells, to monitor their environment and initiate appropriate responses. An important component in signal transduction are protein kinases, which phosphorylate enzymes to modulate their activity and relay information. As another important component, many signaling networks also actively employ messenger molecules to coordinate cellular responses. Messenger molecules include calcium as well as reactive oxygen species (ROS). In particular, the active production of ROS by NADPH oxidases (NOXs), for example in response to pathogen infection, is tightly controlled by phosphorylation and other signals to avoid undesirable side effects.
Question: We wanted to understand the molecular function of the protein kinase cysteine-rich receptor-like protein kinase 2 (CRK2) in thale cress Arabidopsis thaliana. Specifically, we were curious how CRK2 is able to control NOXs, the engines of the extracellular ROS burst.
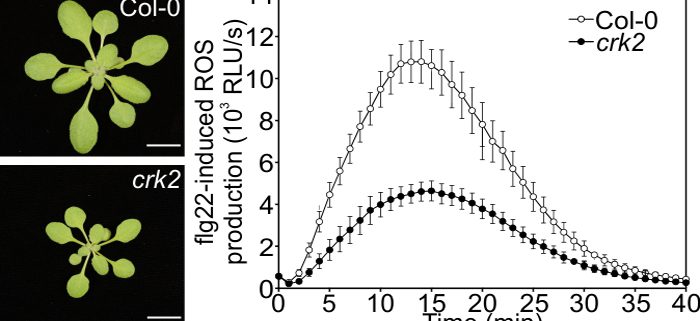
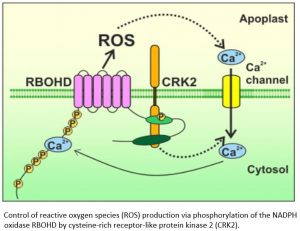 Findings: We found that CRK2 was essential for plant growth and immunity in Arabidopsis. Active CRK2 enhanced the ROS-producing activity of the plant NOX RBOHD. When we disrupted CRK2, ROS production in response to challenge with a pathogen-derived molecule was reduced and consequently plants became more susceptible to the pathogen. This allowed us to investigate the activation mechanism of RBOHD by CRK2. To our surprise, we discovered that, unlike other plant kinases, CRK2 activates RBOHD through phosphorylation of its C-terminus, which is reminiscent of the activation of NOXs in humans. By comparing the amino acid composition of RBOHD with other plant and human NOXs, we were able to propose that regulation of NOX activity by phosphorylation of the C-terminal region might be an ancient mechanism.
Findings: We found that CRK2 was essential for plant growth and immunity in Arabidopsis. Active CRK2 enhanced the ROS-producing activity of the plant NOX RBOHD. When we disrupted CRK2, ROS production in response to challenge with a pathogen-derived molecule was reduced and consequently plants became more susceptible to the pathogen. This allowed us to investigate the activation mechanism of RBOHD by CRK2. To our surprise, we discovered that, unlike other plant kinases, CRK2 activates RBOHD through phosphorylation of its C-terminus, which is reminiscent of the activation of NOXs in humans. By comparing the amino acid composition of RBOHD with other plant and human NOXs, we were able to propose that regulation of NOX activity by phosphorylation of the C-terminal region might be an ancient mechanism.
Next steps: A plethora of signaling elements converge on ROS producing enzymes to regulate their activity. Our work demonstrates that some regulatory components are even conserved between animals and plants. In the future, we need to obtain a better understanding on how the different signals are integrated to allow plants to very precisely control and fine-tune ROS production.
Key words: protein kinase, reactive oxygen species, pathogen
Sachie Kimura, Kerri Hunter, Lauri Vaahtera, Huy Cuong Tran, Matteo Citterico, Aleksia Vaattovaara, Anne Rokka, Sara Christina Stolze, Anne Harzen, Lena Meißner, Maya Melina Tabea Wilkens, Thorsten Hamann, Masatsugu Toyota, Hirofumi Nakagami, Michael Wrzaczek (2020) CRK2 and C-terminal Phosphorylation of NADPH Oxidase RBOHD Regulate Reactive Oxygen Species Production in Arabidopsis. Plant Cell https://doi.org/10.1105/tpc.19.00525