Adenylate cyclase is a critical component of auxin transcriptional responses
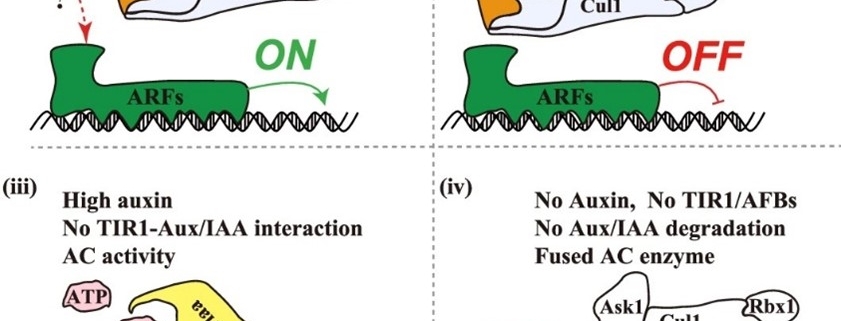
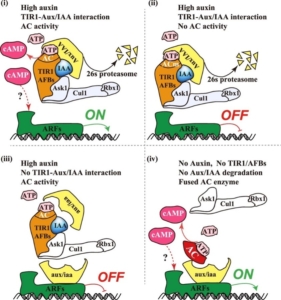 This exciting paper by Chen, Qi, Zou et al. adds a new twist to the story of auxin. Until this work, the current model for how auxin mediates transcriptional changes has been that auxin (IAA) binds to TIR1, a ubiquitin ligase, when interacts with and causes the degradation of Aux/IAA repressor proteins, thereby permitting ARF transcription factors to promote transcription. The new study reveals that TIR1 has auxin-induced adenylate cyclase (AC) activity (which converts ATP to cyclic AMP, cAMP) that is both necessary and sufficient for the auxin response. The authors tested this through several different approaches. They showed that interaction between TIR1 and Aux/IAAs is necessary for the auxin-induced increase in AC activity, but when they knocked out the AC activity, they found that its loss does not affect auxin-induced degradation of Aux/IAAs. However, AC-deficient TIR1 is not able to promote expression of auxin-induced genes. Finally, they showed that AC activity alone in the vicinity of auxin-regulated genes is sufficient to induce their expression. They conclude that cAMP acts as a second messenger essential for transcriptional reprogramming. (Summary by Mary Williams @PlantTeaching.bsky.social) Nature 10.1038/s41586-025-08669-w
This exciting paper by Chen, Qi, Zou et al. adds a new twist to the story of auxin. Until this work, the current model for how auxin mediates transcriptional changes has been that auxin (IAA) binds to TIR1, a ubiquitin ligase, when interacts with and causes the degradation of Aux/IAA repressor proteins, thereby permitting ARF transcription factors to promote transcription. The new study reveals that TIR1 has auxin-induced adenylate cyclase (AC) activity (which converts ATP to cyclic AMP, cAMP) that is both necessary and sufficient for the auxin response. The authors tested this through several different approaches. They showed that interaction between TIR1 and Aux/IAAs is necessary for the auxin-induced increase in AC activity, but when they knocked out the AC activity, they found that its loss does not affect auxin-induced degradation of Aux/IAAs. However, AC-deficient TIR1 is not able to promote expression of auxin-induced genes. Finally, they showed that AC activity alone in the vicinity of auxin-regulated genes is sufficient to induce their expression. They conclude that cAMP acts as a second messenger essential for transcriptional reprogramming. (Summary by Mary Williams @PlantTeaching.bsky.social) Nature 10.1038/s41586-025-08669-w