A three-way dance: NAD-ME switches subunits to perform different tasks
Meike Hüdig, Molecular Plant Physiology, University of Bonn; Marcos A. Tronconi, Centro de Estudios Fotosintéticos y Bioquímicos, Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Argentina. Veronica Maurino, Molecular Plant Physiology, University of Bonn
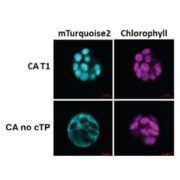
Background: C4 photosynthesis is an efficient improvement of the standard CO2 fixation pathway that evolved over 60 times. This auxiliary metabolic route prevents Rubisco oxygenase activity by enriching CO2 in its surroundings. C4-NAD-malic enzyme (NAD-ME) releases the prefixed CO2 to feed Rubisco in about one-third of these C4 lineages. Furthermore, in every cell, NAD-ME functions in mitochondrial malate respiration, a housekeeping role. Most C4 enzymes originated by gene duplication and subsequent neofunctionalization of one housekeeping gene copy. However, the evolution of C4-NAD-ME showed sub-functionalization and differences in the frequency of gene duplication of the two paralogous α- and β-NAD-ME genes that encode the subunits of NAD-ME. We used two independent C4-NAD-ME lineages from the Cleomaceae (Gynandropsis gynandra, see image, and Cleome angustifolia) and a C3 sister species (Tarenaya hassleriana) to unravel the mystery around the elusive C4-NAD-ME.
Question: Given that the evolution of C4-NAD-ME turned out to be an exceptional case, we wanted to know if there is a specific NAD-ME version to perform this additional task and, if so, how it differs from its housekeeping NAD-ME.
Findings: NAD-ME is comprised of two different α and β protein subunits. In both G. gynandra and C. angustifolia, the β subunit was duplicated (β1 and β2), resulting in two enzymatic entities, NAD-MEα/β1 and NAD-MEα/β2. The C4-decarboxylase role is conducted by the NAD-MEα/β1 heteromer, a highly efficient enzymatic entity exclusively present in photosynthetic tissues. Unlike the β2 subunit, β1 is a non-catalytic protein forming more rigid contacts with the associated α-subunit; it is also responsible for activating C4-NAD-ME by aspartate, an important C4 metabolite. The C4 decarboxylase coexists with the housekeeping NAD-MEα/β2 in mitochondria. In silico simulations suggest that positively selected amino acid substitutions in the β1 subunit of both C4 species enables the differential assembly of the subunits to form independent enzymatic entities acting in the same cell compartment.
Next steps: Our work demonstrates a possible evolutionary route of heteromeric proteins. We want to explore the identified changes in the β subunits to pinpoint the properties to single amino acids. This could help to engineer existing NAD-ME with new C4 properties.
Meike Hüdig, Marcos A. Tronconi, Juan P. Zubimendi, Tammy L. Sage, Gereon Poschmann, David Bickel, Holger Gohlke, Veronica G. Maurino. (2022). Respiratory and C4-photosynthetic NAD-malic enzyme coexist in bundle sheath cell mitochondria and evolved via association of differentially adapted subunits. https://doi.org/10.1093/plcell/koab265