A New Interaction Between Myosin XI and Exocyst in Plant Secretion
Zhang et al. explore the role of myosin XI in exocytosis.
Plant Cell
Weiwei Zhang and Christopher J. Staiger
Purdue University
Background: Living organisms secrete molecules from their cells to allow growth and survival in various environments. Materials destined for secretion are often packed into membrane-bound compartments called vesicles and are delivered to the cell surface or plasma membrane (PM). Exocytosis is the last step of secretory trafficking, which involves tethering, docking and fusion of vesicle membrane with the PM. One essential player in vesicle trafficking and exocytosis is the molecular motor, myosin, that powers various intracellular movements. In yeast and animal cells, myosins drive vesicle transport along actin cable highways, ensuring precise delivery of cargo to their target membrane. Myosins interact with important trafficking-related proteins to regulate this process, including the exocyst complex, a multi-subunit protein complex implicated in vesicle tethering to the PM. In plant cells, myosin has been shown to function in long-distance transport; however, a role in regulating exocytosis has not been discovered.
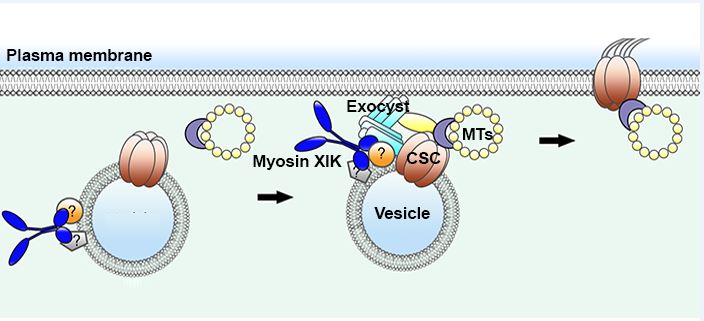
Question: We previously showed that plant myosin XI mediates exocytosis of cellulose synthase complexes (CSCs), complexes that produce cell wall building blocks. Myosin XI was transiently present at the vesicle tethering site and is required for efficient CSC delivery at the PM. We examined the specific role of myosin XI in exocytosis and tested whether it helps regulate vesicle tethering.
Findings: We found that myosin XIK is the primary isoform mediating CSC trafficking in Arabidopsis. Moreover, myosin XIK participates in vesicle tethering through direct interactions with the exocyst complex. Several exocyst subunits directly bind to the globular tail domain of XIK. In live cells, myosin XIK and exocyst subunits not only colocalize but are also functionally related. Further, XIK associates with secretory vesicles earlier than exocyst and is required for the efficient recruitment and stabilization of exocyst complex at the PM tethering site.
Next steps: It remains unclear how myosin XI is recruited and bound to secretory vesicles and whether other players, such as Rab GTPases, are involved in the same interaction network to coordinate vesicle trafficking and secretion in plants. Future work is needed to uncover the functional connection and interaction between myosin motors, exocyst, Rab GTPases and other players in plant cells.
Weiwei Zhang, Lei Huang, Chunhua Zhang and Christopher J. Staiger. (2021). Arabidopsis myosin XIK interacts with the exocyst complex to facilitate vesicle tethering during exocytosis. https://doi.org/10.1093/plcell/koab116