A clustered mitochondria family protein mediates the plant mitophagy (Curr. Biol.)
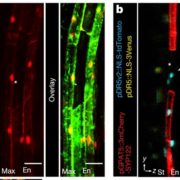
 Mitochondria function as cellular powerhouses to generate energy via oxidative phosphorylation and facilitate the synthesis of essential macromolecules. To protect against proteotoxic stress, damaged mitochondria are selectively removed by autophagy via a process known as mitophagy. In mammalian cells, the selective mitophagy receptors and adaptors proteins have been extensively studied, but those homologs are mostly absent from the plant genome. Previusly, an Arabidopsis mutant named friendly was identified in which mitochondria cluster together; FRIENDLY is a member of the conserved CLUSTERED MITOCHONDRIA family. To study the process of mitophagy, Ma et al. used uncouplers of oxidative phosphorylation such as the proton ionophore DNP to induce mitophagy. Depolarized mitochondria are selectively engulfed by autophagosomes, dependent on ATG5. Uncoupler treatments also induce ubiquitination of the outer mitochondria membrane, involving both mitophagy and proteasome degradation pathways. The authors found the friendly mutant has less mitophagosomes and displays defective elimination of damaged mitochondria, indicating that FRIENDLY is essential for uncoupler-induced mitophagy. Interestingly, the induced mitophagy causes delay of greening cotyledon in the friendly and atg5 mutants, suggesting its role in metabolic switching. This study sheds lights on how plants employ mitophagy in inter-organelle communication under stress and development. (Summary by Min May Wong @wongminmay) Curr. Biol. 10.1016/j.cub.2021.02.034
Mitochondria function as cellular powerhouses to generate energy via oxidative phosphorylation and facilitate the synthesis of essential macromolecules. To protect against proteotoxic stress, damaged mitochondria are selectively removed by autophagy via a process known as mitophagy. In mammalian cells, the selective mitophagy receptors and adaptors proteins have been extensively studied, but those homologs are mostly absent from the plant genome. Previusly, an Arabidopsis mutant named friendly was identified in which mitochondria cluster together; FRIENDLY is a member of the conserved CLUSTERED MITOCHONDRIA family. To study the process of mitophagy, Ma et al. used uncouplers of oxidative phosphorylation such as the proton ionophore DNP to induce mitophagy. Depolarized mitochondria are selectively engulfed by autophagosomes, dependent on ATG5. Uncoupler treatments also induce ubiquitination of the outer mitochondria membrane, involving both mitophagy and proteasome degradation pathways. The authors found the friendly mutant has less mitophagosomes and displays defective elimination of damaged mitochondria, indicating that FRIENDLY is essential for uncoupler-induced mitophagy. Interestingly, the induced mitophagy causes delay of greening cotyledon in the friendly and atg5 mutants, suggesting its role in metabolic switching. This study sheds lights on how plants employ mitophagy in inter-organelle communication under stress and development. (Summary by Min May Wong @wongminmay) Curr. Biol. 10.1016/j.cub.2021.02.034