Uncovering the Steps Before: Sulfate Induces ABA Biosynthesis and Stomatal Closure
Plant stomatal aperture regulation via guard cells is an example of how plants dynamically process environmental signals to induce a physiological response. The drought stress hormone abscisic acid (ABA) is a well-characterized signal that induces stomatal closure, preventing water loss. ABA acts via the PYRABACTIN RESISTANCE (PYR)/PYR LIKE (PYRL)-ABA INSENSITIVE1 (ABI1)-OPEN STOMATA1 (OST1) receptor-phosphatase-kinase core signaling pathway. It induces the activity of NADPH oxidases for the production of reactive oxygen species (ROS), important regulators of stomatal closure, in an OST1-dependent manner. Furthermore, drying of soil induces the root-to-shoot transport of sulfate that in turn promotes stomatal closure (Malcheska et al., 2017).
Batool et al. (2018) explored how upstream signaling cascades involving sulfate and cysteine biosynthesis lead to ABA accumulation. To study sulfate-induced stomatal closure in maize (Zea mays) and Arabidopsis thaliana, sulfate was exogenously applied to epidermal peels or administered via petiole feeding, which resulted in stomatal closure. The authors found that sulfate induced ROS formation in guard cells by activating NADPH oxidases. To explore if sulfate-induced ROS formation and stomatal closure act via the ABA signaling pathway, sulfate was applied to aba3-1, which is impaired in ABA biosynthesis. Whereas the aba3-1 mutant exhibited ROS production and stomatal closure in response to ABA treatment, sulfate treatment did not elicit the same response. Therefore, sulfate-induced ROS production depends on de novo ABA biosynthesis via ABA3. Further, exogenous sulfate treatment increased the intracellular ABA concentration in wild-type guard cells, leading to enhanced transcription of ABA marker genes.
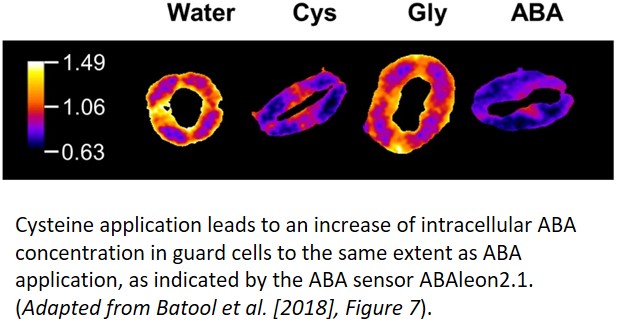
How does sulfur metabolism influence sulfate-induced stomatal closure? The authors used the sir1-1 mutant, which cannot convert sulfite to sulfide, and the serat tko mutant, which is defective in incorporating sulfide into cysteine, to examine this question. Neither of these mutants exhibited stomatal closure or ROS formation in response to sulfate treatment. However, exogenous ABA and cysteine treatment led to stomatal closure in both mutants, indicating a requirement for the metabolic assimilation of sulfate into cysteine by SERAT and SiR for sulfate-induced closure. Furthermore, direct application of cysteine in the wild type also elevated the intracellular ABA concentration (see figure) and induced ROS formation.
The requirement for the incorporation of sulfide into cysteine for sulfate-induced stomatal closure was explored further in the context of ABA biosynthesis. Previous studies showed that ABA3 uses cysteine as a sulfur donor to convert abscisic aldehyde to ABA via ABSCISIC ALDEHYDE OXIDASE 3 (AAO3; Bittner et al., 2001, Cao et al., 2014). Batool et al. extended this line of research by demonstrating that petiole feeding of sulfate or cysteine induced the transcription of NINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3 (NCED3), which limits the synthesis of the AAO3 substrate and consequently ABA biosynthesis. However, cysteine and sulfate application did not induce stomatal closure in the aba3-1 and nced3-2 loss-of-function mutants, indicating that both NCED and ABA3 activity are required for cysteine- and sulfate-induced stomatal closure.
Cysteine synthesis-depleted mutants were found to have enhanced wilting and suffered lower relative water content than wild-type plants under limited water supply. This reflects the need for adequate cysteine to promote decreased transpiration in leaves when soil drying occurs, most likely due to the limited capability of being able to synthesize ABA to lead to stomatal closure.
Batool et al. demonstrate that sulfate promotes stomatal closure by inducing ABA biosynthesis and activating ROS. The incorporation of sulfate into cysteine leads to ABA biosynthesis in leaves, highlighting how sulfate can act as a limiting factor to initiate the hormone signaling pathway important for stomatal closure in plants under stress.
REFERENCES
Batool S, Uslu V V, Rajab H, Ahmad N, Waadt R, Geiger D, Malagoli M, Xiang C-B, Hedrich R, Rennenberg H, Herschbach C, Hell R, and Wirtz M. (2018). Sulfate is incorporated into cysteine to trigger ABA production and stomata closure. Plant Cell 10.1105/tpc.18.00612.
Bittner F, Oreb M, and Mendel R R. (2001). ABA3 Is a Molybdenum Cofactor Sulfurase Required for Activation of Aldehyde Oxidase and Xanthine Dehydrogenase in Arabidopsis thaliana. Journal of Biological Chemistry 276, 40381-40384.
Cao M J, Wang Z, Zhao Q, Mao J L, Speiser A, Wirtz M, Hell R, Zhu J K, and Xiang C B. (2014). Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant Journal 77, 604-615.
Malcheska F et al. (2017). Drought-Enhanced Xylem Sap Sulfate Closes Stomata by Affecting ALMT12 and Guard Cell ABA Synthesis. Plant Physiology 174, 798-814.