Twin-Positive Motifs Function as Specific Plastid Targeting Signals
Precise trafficking of proteins to their proper destinations in the cell, whether to an organelle, a membrane, or the cytoplasm, is required for optimal cellular function. Because most plastid proteins are nucleus-encoded and translated in the cytoplasm, proper targeting and import relies on the presence of specific N-terminal transit peptide sequences. But what signals direct a particular preprotein to a certain type of plastid? An N-terminal domain containing multiple Arg and hydrophobic residues was identified as a mitochondrial specificity factor (Lee et al., 2019), and consecutive positively charged (twin-positive) residues were shown to be important for preprotein import into old vs. young chloroplasts (Teng et al., 2012).
 Recent work by Chu et al. (2020) reveals particular transit peptide motifs that confer tissue-specific import of preproteins into leucoplasts vs. chloroplasts. Using an in vitro system they have optimized for analyzing protein import into pea root leucoplasts, the authors compared the import efficiency of the homologous preproteins of plastoglobulin 35 (prPGL35) and fibrillin 1B (prFB) into leucoplasts vs. chloroplasts. Both proteins were imported equally well into chloroplasts, but prFB was more efficiently imported into leucoplasts. By replacing regions of the prPGL35 transit peptide with corresponding sequences from prFB, they found two regions of prFB that improved import of prPGL35 into leucoplasts by more than two-fold: an Arg-Val-Ser-Ile (RVSI) sequence at positions 15-18 and an Arg-Val (RV) sequence at positions 53-54. Mutation of either the RVSI sequence to 4x Ala or the RV sequence to 2x Glu severely reduced prFB import into leucoplasts, but had minimal effect on chloroplast import.
Recent work by Chu et al. (2020) reveals particular transit peptide motifs that confer tissue-specific import of preproteins into leucoplasts vs. chloroplasts. Using an in vitro system they have optimized for analyzing protein import into pea root leucoplasts, the authors compared the import efficiency of the homologous preproteins of plastoglobulin 35 (prPGL35) and fibrillin 1B (prFB) into leucoplasts vs. chloroplasts. Both proteins were imported equally well into chloroplasts, but prFB was more efficiently imported into leucoplasts. By replacing regions of the prPGL35 transit peptide with corresponding sequences from prFB, they found two regions of prFB that improved import of prPGL35 into leucoplasts by more than two-fold: an Arg-Val-Ser-Ile (RVSI) sequence at positions 15-18 and an Arg-Val (RV) sequence at positions 53-54. Mutation of either the RVSI sequence to 4x Ala or the RV sequence to 2x Glu severely reduced prFB import into leucoplasts, but had minimal effect on chloroplast import.
The introduction of RV into prPGL35 was adjacent to another Arg residue, creating a twin-positive motif. Likewise, mutation of Pro-50 to Arg (P50R) in prPGL35 created a twin-positive motif in the transit peptide, and this change increased import efficiency of prPGL35 into leucoplasts by more than three-fold. They verify the importance of this twin-positive motif by analyzing a set of Ala scanning mutants of prTic40, a preprotein with very high leucoplast import efficiency. Introduction of Ala residues at positions 10-18 in the transit peptide inhibited import into both leucoplasts and chloroplasts. By contrast, mutation of the twin-positive motif (RK29-30 to AA) reduced import into leucoplasts by 80%, while causing only a 20% reduction in chloroplast import. Furthermore, introduction of these two positively charged residues into a defective Ala (AAAA28-31) scanning mutant restored import into leucoplasts, boosting efficiency by 5- to 6-fold.
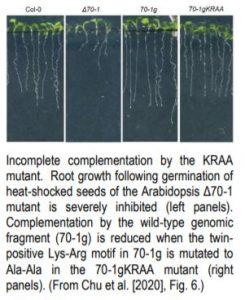
Chloroplast cpHsc70-1, a heat-shock protein important for plastid biogenesis and protein import into plastids in both roots and shoots, has a single twin-positive motif (Lys-Arg) in its transit peptide. When heat-shocked seeds were germinated on MS medium, deletion mutants lacking cpHsc70-1 (Δ70-1) produced severely stunted leaves and roots compared to Col-0 (see figure, left two panels). When the cpHsc70-1 twin-positive residues in a genomic fragment (70-1g) that restored normal root and leaf growth were mutated to Ala (70-1gKRAA), import into leucoplasts, but not chloroplasts, was severely reduced, and root growth was significantly inhibited (right two panels).
To test whether presence of a twin-positive motif in transit peptides correlates with a greater protein or RNA abundance in roots, the authors examined root expression and relative RNA levels in pairs of Arabidopsis preproteins differing in the number of twin-positive motifs in the transit peptide. Their results supported the hypothesis that the twin-positive motif is important for specific leucoplast import. Future work will focus on identifying the leucoplast receptor for the twin-positive motif through biochemical crosslinking and genetic screening for mutants with the goal of increasing our understanding of tissue-specific protein import into plastids and how this contributes to the unique proteomes of particular plastids.
Gregory Bertoni
Science Editor
gbertoni@aspb.org
ORCID ID: 0000-0001-7977-3724
REFERENCES
Chu, C.C., Swamy, K., and Li, H.m. (2020). Tissue-specific regulation of plastid protein import is implemented through transit-peptide motifs. Plant Cell. https://doi.org/10.1105/tpc.19.00702
Lee, D.W., Lee, S., Lee, J., Woo, S., Razzak, M.A., Vitale, A., and Hwang, I. (2019). Molecular mechanism of the specificity of protein import into chloroplasts and mitochondria in plant cells. Molec. Plant 12: 951-966.
Teng, Y.S., Chan, P.T., and Li, H.m. (2012). Differential age-dependent import regulation by signal peptides. PLoS Biol. 10: e1001416.



