Too Much, Take it Back: PAP Moves from the Cytosol to Plastids and Mitochondria for Degradation via PAPST2
Plants need to strike a fine balance between the production, transport, and degradation of stress signals in order to optimize growth in fluctuating environmental conditions. The signal 3’phosphoadenosine 5’phosphate (PAP) accumulates during drought and light stress and induces stress-responsive gene expression (Estavillo et al., 2011). Sulfur transfer from 3’phosphoadenosine 5’phosphosulfate (PAPS) to acceptor compounds in the cytosol results in the release of PAP. PAP is then degraded by the enzyme SAL1 in plastids and mitochondria into AMP and inorganic phosphate. SAL1 tightly controls PAP levels, as constitutively elevated PAP levels markedly affect growth and development (Estavillo et al., 2011). However, insights into how this signaling molecule moves between different cellular compartments, and the specific implications of subcellular concentrations, have been elusive. Ashykhmina et al. (2018) expand on the discovery of the plastid-specific PAPS transporter PAPST1 (Gigolashvili et al., 2012) and define the role of the closest homolog, PAPST2, as a key component of the PAP-degradation and signaling pathway in Arabidopsis thaliana.
The authors heterologously expressed and reconstituted PAPST2 in artificial lipid vesicles to biochemically characterize the transporter. As PAPST1 has previously been classified as an antiporter, which can concurrently move different nucleotides in opposite directions (Gigolashvili et al., 2012), the vesicles were loaded with ATP and different substrates were tested. Compared to PAPST1, which preferentially transports PAPS and ATP over PAP, PAPST2 was found to efficiently transport PAP, PAPS, ATP, and ADP, albeit with a slight preference for PAP and ATP. Furthermore, PAPST2 was found to be dually targeted to chloroplasts and mitochondria.
Considering that elevated PAP levels lead to plant developmental defects, as exhibited by the SAL1 loss-of-function mutant fry1 (Estavillo et al., 2011), PAP-transport mutants were morphologically characterized. papst2 T-DNA and amiRNA mutant lines have a larger rosette than wild type and papst1 plants. Both palisade and spongy parenchyma cells were enlarged in papst2 T-DNA mutants.
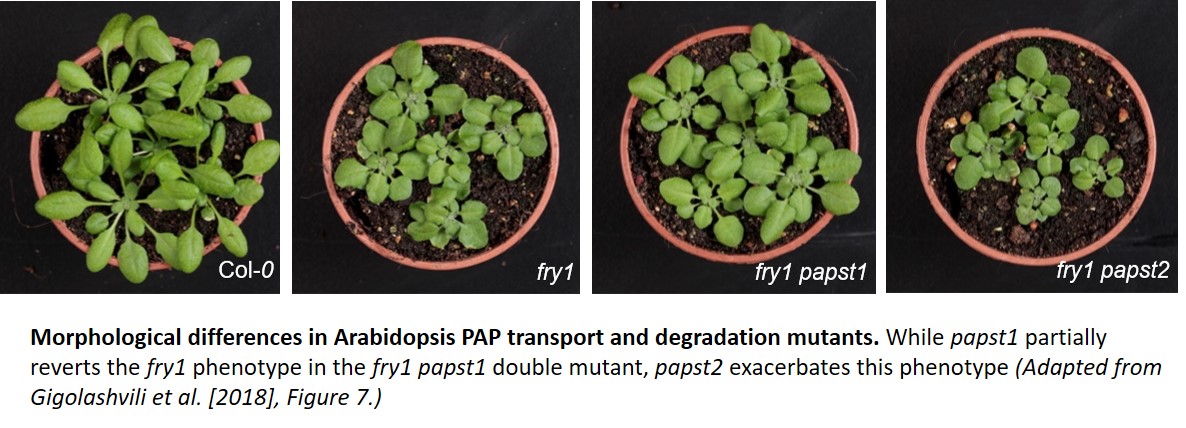
Are these morphological differences due to changes in organellar PAP levels? papst2 had a moderate increase in cytosolic PAP levels compared to wild type, thereby stimulating plant growth, as exemplified in papst2 and amiRNA lines. As the fry1 mutant has far higher PAP levels and is smaller than wild type, it was crossed with papst1 and papst2. The morphological traits of fry1 were partially complemented in the fry1 papst1 double mutant (see figure), and this coincided with lowered cytosolic PAP content but higher chloroplastic PAP content compared to fry1 alone. This could potentially be due to an overall decrease in PAPS transport via PAPST1, and consequently, lower PAP formation in the cytosol. By contrast, fry1 papst2 had an exacerbated fry1 phenotype, with higher cytosolic and chloroplastic PAP contents compared to fry1.
As SAL1 is important for the degradation of PAP and is dually localized to mitochondria and chloroplasts, organelle-specific complementation was explored. Dual and mitochondrial SAL1 lowered PAP accumulation in fry1 papst1 to wild-type levels, suggesting that mitochondrial SAL1 can detoxify PAP, provided mitochondrial import of PAP is still functional (i.e., via PAPST2). In the fry1 papst2 mutant, expression of a dually targeted SAL1 led to normal plant growth and a reduction in cellular PAP concentration similar to wild-type levels. However, PAP levels remained high and mutants were still morphologically small when SAL1 was targeted to the mitochondria in fry1 papst2 plants. This indicates that PAPST2 is the central transporter that fuels mitochondrial SAL1 and, consequently, mitochondrial PAP degradation.
PAPST1, PAPST2, and SAL1 coordinate PAP levels via transport and degradation between different cellular compartments. Ashykhmina et al. provide evidence that PAPST2 is important for cytosolic PAP removal and mitochondrial PAP uptake. Detrimental morphological phenotypes are associated with high levels of PAP in the cytosol, but small increases could potentially enhance growth. These findings implicate PAPST2 and PAP transport as key components in PAP degradation and potentially the PAP signaling pathway.
REFERENCES
Ashykhmina N, Lorenz M, Frerigmann H, Koprivova A, Hofsetz E, Stührwohldt N, Flügge U I, Haferkamp I, Kopriva S and Gigolashvili T. (2018). PAPST2 plays a critical role in PAP removal from the cytosol and subsequent degradation in plastids and mitochondria. Plant Cell 10.1105/tpc.18.00512.
Estavillo, GM et al (2011). Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23, 3992-4012.
Gigolashvili T, Geier M, Ashykhmina N, Frerigmann H, Wulfert S, Krueger S, Mugford S G, Kopriva S, Haferkamp I, and Flügge U-I. (2012). The Arabidopsis thylakoid ADP/ATP carrier TAAC has an additional role in supplying plastidic phosphoadenosine 5′-phosphosulfate to the cytosol. Plant Cell 21, 4187-4204.



