The protein phosphatase PP2A-B’γ takes control over salicylic acid to suppress defense and premature senescence
Amna Mhamdi
Ghent University, Department of Plant Biotechnology and Bioinformatics, and VIB Center for Plant Systems Biology, 9052 Ghent, Belgium
Address correspondence to [email protected]
Long thought to be unselective and often referred to as “housekeeping” enzymes, type 2A Protein Phosphatases (PP2A) have emerging specific regulatory functions in cell signaling (Luan, 2003; Durian et al., 2016; Máthé et al., 2019). PP2As interact with several pathways to activate signal transduction and adaptive responses while preventing unnecessary investment of energy.
PP2A is a heterotrimer, with the core enzyme consisting of a scaffold A subunit, a catalytic C subunit, and a regulatory and variable B subunit with at least 17 isoforms in Arabidopsis (Arabidopsis thaliana). PP2A-B’γ acts as a negative regulator of pathogenesis responses and controls day length-dependent responses to intracellular oxidative stress (Trotta et al., 2011; Li et al., 2014; Segonzac et al., 2014). However, how this phosphatase affects plant metabolism and defense remains to be established, simply because only a few targets of PP2A-B’γ have been identified.
In this issue of Plant Physiology, Durian et al. (2020) define the mechanisms by which PP2A-B’γ prevents defense responses and delays senescence (Figure 1). First, the authors provide evidence for an interaction between PP2A-B’γ and CALCIUM-DEPENDENT PROTEIN KINASE 1 (CPK1), a kinase that positively regulates defense against the necrotrophic fungal pathogen Botrytis cinerea (Coca and San Segundo, 2010). Under control conditions, pp2a-b´γ mutants displayed increased in-gel kinase activity of CPK1. Treatment with Botrytis increased CPK1 abundance and this was particularly strong in the pp2a-b´γ background (Durian et al., 2020). Even though CPK1 was previously implicated in salicylic acid (SA)-related gene expression in response to Fusarium oxysporum, in this study analysis of cpk1 mutants revealed that CPK1 is not required for accumulation of PATHOGENESIS RELATED1 (PR1) proteins after Botrytis infection.
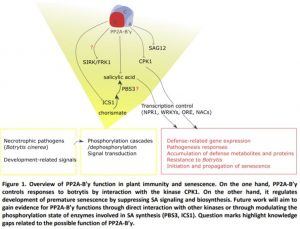 A second mechanism by which PP2A-B’γ controls disease resistance and the development of leaf senescence involves suppressing SA signaling. Transcriptome and metabolite profiling demonstrated that pp2a-b´γ mutants are primed for stress and accumulate markers of defense, in particular SA, camalexin and SA-related transcripts including PR1, PR2, and PR5, and WRKY transcription factors (Durian et al., 2020). Consistent with this, the pp2a-b´γ mutants exhibited premature leaf senescence in the apical parts of leaves, associated with accumulation of PR1 and SENESCENCE ASSOCIATED GENE 12 proteins. Durian and colleagues demonstrate that the premature leaf yellowing phenotype requires SA biosynthesis via SALICYLIC ACID INDUCTION DEFICIENT2/ISOCHORISMATE SYNTHASE1 (SID2/ICS1). Furthermore, PP2A-B’γ function interferes with SA signaling through NONEXPRESSER OF PR GENES 1 (Figure 1).
A second mechanism by which PP2A-B’γ controls disease resistance and the development of leaf senescence involves suppressing SA signaling. Transcriptome and metabolite profiling demonstrated that pp2a-b´γ mutants are primed for stress and accumulate markers of defense, in particular SA, camalexin and SA-related transcripts including PR1, PR2, and PR5, and WRKY transcription factors (Durian et al., 2020). Consistent with this, the pp2a-b´γ mutants exhibited premature leaf senescence in the apical parts of leaves, associated with accumulation of PR1 and SENESCENCE ASSOCIATED GENE 12 proteins. Durian and colleagues demonstrate that the premature leaf yellowing phenotype requires SA biosynthesis via SALICYLIC ACID INDUCTION DEFICIENT2/ISOCHORISMATE SYNTHASE1 (SID2/ICS1). Furthermore, PP2A-B’γ function interferes with SA signaling through NONEXPRESSER OF PR GENES 1 (Figure 1).
The report by Durian et al., (2020) enhances our understanding of how PP2A shapes the phosphoproteome to control plant senescence and illustrates how a regulatory subunit has evolved to regulate specifically one immune signal, SA. During plant development, PP2A-B’γ takes control over SA biosynthesis and signaling to suppress immune functions in young plants and inhibit the initiation and progression of senescence in mature leaves of older plants.
However, we are only looking at the tip of the iceberg, as phosphatase substrate identification is still a tough task for researchers working in this field. Several key questions remain about how this phosphatase functions. For example, does PP2A-B’γ regulatory role require another kinase-phosphatase interaction to control SA-dependent early senescence (e.g. interaction with SENESCENCE-INDUCED RECEPTOR-LIKE KINASE, SIRK/FRK1 (Asai et al., 2002), a kinase involved in early defense signaling? Or does it act on SA synthesis by dephosphorylating AvrPphB SUSCEPTIBLE3, PBS3; (Rekhter et al., 2019) a cytosolic enzyme recently shown to catalyze the last step of isochorismate conversion to SA?
References
Asai T, Tena G, Plotnikova J, Willman, MR, Chiu W, Gómez‐Gómez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 415: 977– 983.
Coca M, San Segundo B (2010) AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J. 63: 526–540
Durian G, Rahikainen M, Alegre S, Brosché M, Kangasjärvi S (2016) Protein Phosphatase 2A in the regulatory network underlying biotic stress resistance in plants. Front Plant Sci 7: 812
Durian G, Jeschke V, Rahikainen M, Vuorinen K, Gollan PJ, Brosché M, Salojärvi J, Glawischnig E, Winter Z, Li S, Noctor G, Aro EM, Kangasjärvi J, Overmyer K, Burow M, Kangasjärvi S (2020) PROTEIN PHOSPHATASE 2A-B′γ controls Botrytis cinerea resistance and developmental leaf senescence. Plant Physiol. DOI:10.1104/pp.19.00893 {https://doi.org/10.1104/pp.19.00893}
Li S, Mhamdi A, Trotta A, Kangasjärvi S, Noctor G (2014) The protein phosphatase subunit PP2A-B′γ is required to suppress day length-dependent pathogenesis responses triggered by intracellular oxidative stress. New Phytol 202: 145–160
Luan S (2003) Protein phosphatases in plants. Annu. Rev. Plant Biol. 54:63–92
Máthé C, Garda T, Freytag C and M-Hamvas M (2019) The role of serine-threonine Protein Phosphatase PP2A in plant oxidative stress signaling-facts and hypotheses. Int. J. Mol. Sci. 20: 3028 doi:10.3390/ijms20123028
Rekhter D, Lüdke D, Ding Y, Feussner K, Zienkiewicz K, Lipka V, Wiermer M, Zhang Y, Feussner I (2019) Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 365: 498–502
Segonzac C, Macho AP, Sanmartín M, Ntoukakis V, Sánchez-Serrano JJ, Zipfel C (2014) Negative control of BAK1 by protein phosphatase 2A during plant innate immunity. Eur Mol Biol Organ J 33: 2069–2079
Trotta A, Wrzaczek M, Scharte J, Tikkanen M, Konert G, Rahikainen M, Holmstrom M, Hiltunen H-M, Rips S, Sipari N, et al (2011) Regulatory subunit B’ of Protein Phosphatase 2A prevents unnecessary defense reactions under low light in Arabidopsis. Plant Physiol 156: 1464–1480