Taking a closer look at cellulose biosynthesis in plants
By Sydney G. Duncombe, Samir Grama Chethan, Charles T. Anderson, Department of Biology, The Pennsylvania State University, University Park, PA 16802 USA
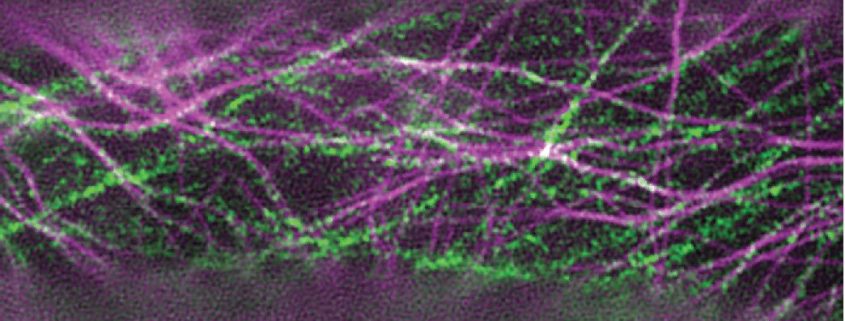
Background: Cellulose is one of the main components of plant cell walls and is made by cellulose synthase (CESA) protein complexes that work together to produce a cellulose microfibril. CESA complexes labeled with fluorescent tags can be observed in living cells by microscopy as they move through the plasma membrane, often along microtubule tracks. Although we have learned much about CESA behavior from conventional light microscopy, the associated resolution limit constrains what we can learn about these tiny molecular machines. To improve our understanding of how CESA complexes behave, we used super-resolution microscopy to improve resolution two-fold over confocal microscopy, allowing us to make new observations about how cellulose, the most abundant biopolymer in nature, is made.
Question: We wanted to improve imaging resolution to learn more about CESA complexes and their behavior in living plant cells. Based on prior high-resolution electron microscopy data collected from dead cells, we suspected that the plasma membrane of living cells contained more CESA particles than could be resolved with confocal microscopy.
Findings: Super-resolution images of fluorescently tagged CESAs showed twice as many particles per area as confocal data, implying that CESA particles imaged by confocal microscopy can contain more than one protein complex. Most CESA particles tracked using super-resolution microscopy moved at steady speeds along straight or slightly curved trajectories, but a few CESA particles made U turns, a behavior that was previously unobserved. Last, we found that variation in cell-wide patterning of CESA particles in cotyledon petiole cells does not correspond to differences in cell shape, calling into question the paradigm that cellulose deposition patterns dominantly determine cell shape in plants.
Next steps: Super-resolution microscopy has proven useful for imaging CESAs as the primary cell wall is laid down in growing cells, so we also plan to image CESAs in cells producing secondary cell walls to learn more about how cellulose is produced in these thickened walls. The discovery of U turns will help us study the interactions between CESAs and microtubules as well as how and why they disassociate and associate with each other.
Reference:
Sydney G. Duncombe, Samir Grama Chethan, Charles T. Anderson (2021). Super-resolution imaging illuminates new dynamic behaviors of cellulose synthase. Plant Cell, https://doi.org/10.1093/plcell/koab227