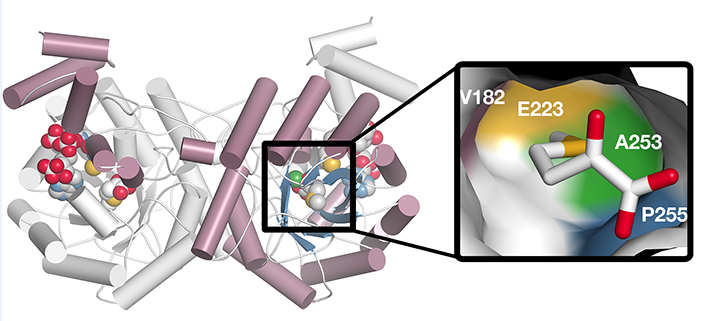
Structure of Methylthioalkylmalate Synthase
Kumar et al. uncover the molecular basis for the diversification of the agriculturally important methionine-derived glucosinolates in plants. Plant Cell https://doi.org/10.1105/tpc.19.00046
Background: The chemical diversity of specialized metabolites in plants helps them to better adapt and survive in a variety of environments. Glucosinolates are amino-acid derived molecules primarily found in plants of the order Brassicales, which includes agriculturally important Brassica crops and the model plant Arabidopsis thaliana. The biosynthesis of aliphatic glucosinolates derived from methionine involves three steps: side-chain elongation, core structure formation, and side-chain modification. Variation in these reactions leads to the chemical diversity of the nearly 130 glucosinolates identified to date. This diversity requires a set of enzymes that alter the length of the possible products of the pathway and modifications of the core chemical structure. Methylthioalkylmalate synthase (MAMS) catalyzes the side-chain elongation of methionine-derived aliphatic glucosinolates and is considered to be the ‘gatekeeper’ enzyme to the elongation process.
Question: Globally cultivated Brassica species possess diverse aliphatic glucosinolates important for plant defense and animal nutrition. The molecular basis for the evolution of MAMS and its generation of natural product diversity is poorly understood in Brassicaceae, owing to their polyploidization and neo-functionalization of paralogs during evolution.
Findings: Our results provide the evolutionary and mechanistic foundation for diversification of different glucosinolate profiles across globally cultivated Brassica crops. We show that the oilseed Brassica juncea genome encodes multiple MAMS that fall into two sub-clades with different expression profiles and substrate preferences. In vitro and in vivo work indicates that two related MAMS catalyze the extension of longer 2-oxoacid substrates and the other two enzymes are restricted to shorter 2-oxoacid substrates. X-ray crystallography of MAMs revealed the key amino acid changes that dictate specificity for different sized 2-oxoacid substrates. Biochemical studies show that a handful of changes in the MAMS active site can alter glucosinolate profiles in Brassica crops.
Next steps: The manipulation of beneficial glucosinolate compounds for animal health and plant protection is a major breeding objective in Brassica crops. Our work provides structural and biochemical insights into the key checkpoints shared in the committed steps of primary (i.e., leucine) and specialized (i.e., glucosinolate) metabolic pathways, which can be extrapolated to selectively manipulate nutritionally favored metabolites in crop plants.
Roshan Kumar, Soon Goo Lee, Rehna Augustine, Micheal Reichelt, Daniel G. Vassão, Manoj H. Palavalli, Aron Allen, Jonathan Gershenzon, Joseph M. Jez*, and Naveen C. Bisht* (2019). Molecular Basis of the Evolution of Methylthioalkylmalate Synthase and the Diversity of Methionine-Derived Glucosinolates. Plant Cell 31: xxx; DOI: https://doi.org/10.1105/tpc.19.00046
*Corresponding authors: [email protected]; [email protected].
Key words: Glucosinolates, Methylthioalkylmalate Synthase (MAMS), primary and secondary metabolism, structure-function relationship, neo-functionalization