Structure and activation of a plant NLR resistosome ($) (Science)
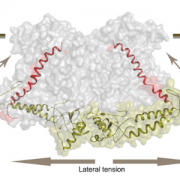
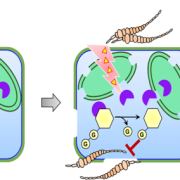
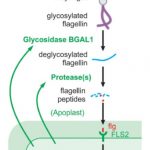
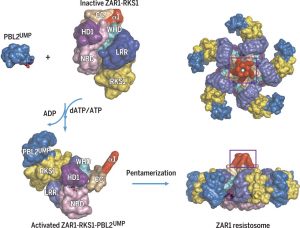 The 2017 Chemistry Nobel Prize went to the developers of cryo-electron microscopy, and this method is transforming plant science as well as other life sciences. In a pair of back-to-back papers, Wang et al. used cryo-EM to examine how ZAR1, a CC-NLR protein [Coiled-coil (CC) nucleotide-binding (NB), leucine-rich repeat (LRR)], interacts with its partners and targets in mounting resistance. The authors examine the structures of ZAR1 in various states of activation and with various partners, and examine how removal of various domains affects activity in vivo. Not unexpectedly, the nucleotide binding domain binds ADP or ATP, with nucleotide exchange controlling its activitation, and the LRRs are involved in pentamerization and active complex formation. In the pentamer, the coiled-coil (CC) domains form a central helical barrel that is critical for function and that may interact with the membrane. These studies also allow the authors to compare the plant resistome to various animal defense complexes including apoptosomes and inflammasomes. The work presented is a remarkable and exciting achievement that opens doors to new insights into how the plant immune system functions. (Summary by Mary Williams). Science 10.1126/science.aav5870 and 10.1126/science.aav5868; see also 10.1126/science.aax0174 for a Perspective.
The 2017 Chemistry Nobel Prize went to the developers of cryo-electron microscopy, and this method is transforming plant science as well as other life sciences. In a pair of back-to-back papers, Wang et al. used cryo-EM to examine how ZAR1, a CC-NLR protein [Coiled-coil (CC) nucleotide-binding (NB), leucine-rich repeat (LRR)], interacts with its partners and targets in mounting resistance. The authors examine the structures of ZAR1 in various states of activation and with various partners, and examine how removal of various domains affects activity in vivo. Not unexpectedly, the nucleotide binding domain binds ADP or ATP, with nucleotide exchange controlling its activitation, and the LRRs are involved in pentamerization and active complex formation. In the pentamer, the coiled-coil (CC) domains form a central helical barrel that is critical for function and that may interact with the membrane. These studies also allow the authors to compare the plant resistome to various animal defense complexes including apoptosomes and inflammasomes. The work presented is a remarkable and exciting achievement that opens doors to new insights into how the plant immune system functions. (Summary by Mary Williams). Science 10.1126/science.aav5870 and 10.1126/science.aav5868; see also 10.1126/science.aax0174 for a Perspective.