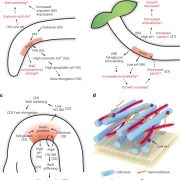
SPY catalyzes O-fucosylation of hundreds of proteins to promote sugar-dependent plant growth
Bi, Shrestha, Zhang et al. elucidate mechanisms of sugar-dependent growth regulation.
Yang Bi, Zhi-Yong Wang
Department of Plant Biology, Carnegie Institution for Science, Stanford, CA, USA
Background: Extensive studies in animals have shown that the O-linked N-acetyl-glucosamine (O-GlcNAc) posttranslational modification of thousands of nucleocytoplasmic proteins, catalyzed by O-GlcNAc transferase (OGT), plays essential roles as a nutrient sensing mechanism. In Arabidopsis thaliana, SPINDLY (SPY) and SECRET AGENT (SEC) are homologous OGTs that catalyze protein O-fucosylation and O-GlcNAcylation, respectively. Loss of SPY causes various developmental defects, and loss of both SPY and SEC causes lethality, indicating overlapping functions. Studies of several O-fucosylated proteins have shown functions of SPY-mediated O-fucosylation in regulating protein activity, supporting the hypothesis that SPY transduces sugar signals to modulate growth and development according to sugar availability.
Question: Does SPY mediate sugar responses or sugar-dependent growth? How can we identify O-fucosylated proteins? What proteins are O-fucosylated and what cellular functions may be regulated by SPY? Is SPY the only or major O-fucosyltransferase in plants? What are the relationships between SPY/O-fucosylation and other sugar signaling pathways?
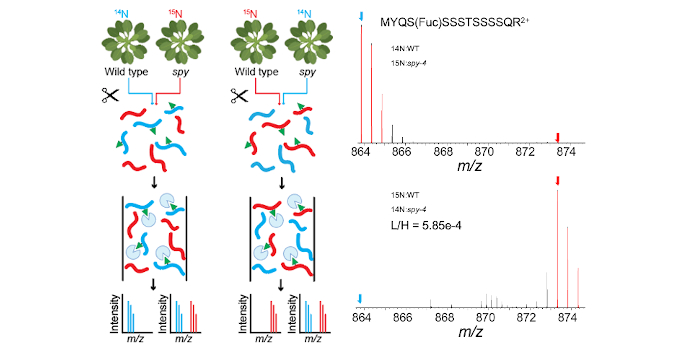
Findings: We show that SPY is required for sugar-dependent growth. We demonstrate an effective lectin-affinity purification method for identifying O-fucosylated proteins by mass spectrometry. We provide evidence that SPY is responsible for all terminal protein O-fucosylation events in Arabidopsis. We identify 467 O-fucosylated Arabidopsis proteins, which are mostly nuclear proteins with important regulatory functions. Our dataset identifies numerous cellular targets of SPY/O-fucosylation and uncovers overlaps with the O-GlcNAc and target of rapamycin (TOR) nutrient-sensing pathways. Our study lays the foundation for functional studies of the SPY/O-fucosylation pathway and the nutrient signaling networks that regulate plant growth and development.
Next steps: How O-fucosylation affects the functions of various proteins is a key question to be addressed in future studies. Conditional loss-of-function spy and sec mutants, as well as tools that alter O-fucosylation or O-GlcNAcylation of individual proteins, will be essential for such functional studies.
Yang Bi, Ruben Shrestha, Zhenzhen Zhang, Chuan-Chih Hsu, Andres V Reyes, Sumudu Karunadasa, Peter R Baker, Jason C Maynard, Yang Liu, Amirmansoor Hakimi, Daniel Lopez-Ferrer, Tahmid Hassan, Robert J Chalkley, Shou-Ling Xu, Zhi-Yong Wang. (2023). SPINDLY mediates O-fucosylation of hundreds of proteins and sugar-dependent growth in Arabidopsis. https://doi.org/10.1093/plcell/koad023