Soybean ACBPs Sort out the Salt with Oxylipins
By Shiu-Cheung Lung and Mee-Len Chye, The University of Hong Kong, Pokfulam, Hong Kong, China
Background: High salt in soil challenges the performance of many crop plants including soybean. Plants adapt to salt stress by modifying their root architecture, generating ion pumps and accumulating metabolites, etc. A better understanding of the mechanisms triggering acclimation will provide strategies for improving tolerance to salinity. One of the salt-associated signaling events involves the production of a second messenger, phosphatidic acid (PA). In Arabidopsis, PA generation from membrane phospholipids is facilitated by a Class II member of the acyl-CoA-binding protein (ACBP) family. ACBPs, which are conserved in virtually all eukaryotes and some pathogenic prokaryotes, bind acyl-CoA esters, the activated form of fatty acids (FAs). Besides PA, another class of lipid-based stress signals, termed oxylipins, are generated by lipoxygenases (LOXs) that catalyze the oxygenation of polyunsaturated FAs (linoleic and linolenic acids).
Question: How are LOXs activated to generate oxylipin signals in response to soil salinity?
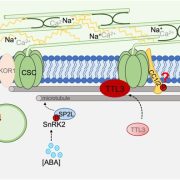
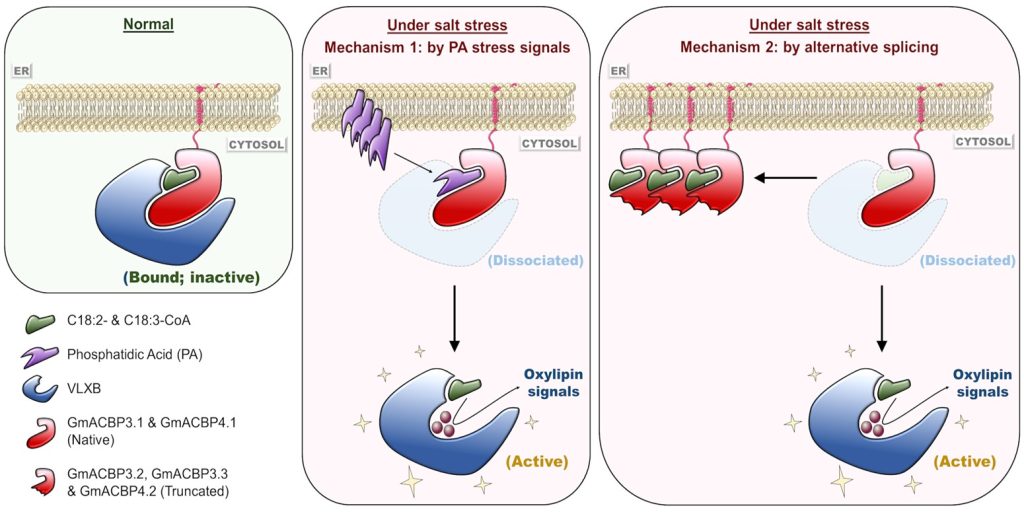 Findings: We found that Class II ACBPs play a central role in eliciting oxylipin signaling in soybean roots during the salinity response. Under normal conditions, binding of these ACBPs with linoleoyl- and linolenoyl-CoAs is critical to mediate protein–protein interaction with LOXs, thereby suppressing the activities of these enzymes by sequestering them at the endoplasmic reticulum membrane. High salinity induces the dissociation of this complex by a dual mechanism. First, specific PA signals are generated and compete with acyl-CoAs for the same lipid-binding site on Class II ACBPs. Second, alternative splicing produces ACBP variants lacking the protein–protein interacting domain, which compete with the native proteins for the same ligands. Release of linoleoyl- and linolenoyl-CoAs weakens the interaction of Class II ACBPs with LOXs, which are subsequently liberated to generate oxylipin signals.
Findings: We found that Class II ACBPs play a central role in eliciting oxylipin signaling in soybean roots during the salinity response. Under normal conditions, binding of these ACBPs with linoleoyl- and linolenoyl-CoAs is critical to mediate protein–protein interaction with LOXs, thereby suppressing the activities of these enzymes by sequestering them at the endoplasmic reticulum membrane. High salinity induces the dissociation of this complex by a dual mechanism. First, specific PA signals are generated and compete with acyl-CoAs for the same lipid-binding site on Class II ACBPs. Second, alternative splicing produces ACBP variants lacking the protein–protein interacting domain, which compete with the native proteins for the same ligands. Release of linoleoyl- and linolenoyl-CoAs weakens the interaction of Class II ACBPs with LOXs, which are subsequently liberated to generate oxylipin signals.
Next steps: We plan to explore the potential of enhancing salt tolerance in soybean by a genetic engineering approach and to uncover other components of the oxylipin signaling mechanism during the salinity response.
Shiu-Cheung Lung, Sze Han Lai, Haiyang Wang, Xiuying Zhang, Ailin Liu, Ze-Hua Guo, Hong-Ming Lam, Mee-Len Chye. (2021). Oxylipin signaling in salt-stressed soybean is modulated by ligand-dependent interaction of Class II acyl-CoA-binding proteins with lipoxygenase. Plant Cell DOI: https://doi.org/10.1093/plcell/koab306