Review. Twenty years of CBL-CIPKs: The knowns and the unknowns (Trends Plant Sci)
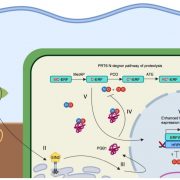
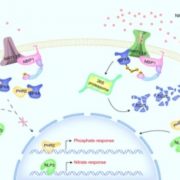
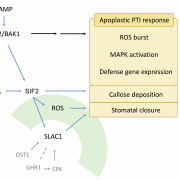
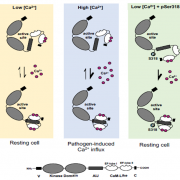
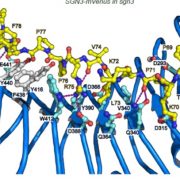
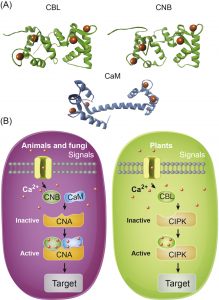 What are the chances that homologs of a vital animal protein could be found in plants, and how much structural and functional similarity would they share? These questions led to the discovery of Calcineurin B-like (CBL) and CBL-interacting protein kinase (CIPK) protein families in plants. In this review, Tang and co-workers discuss how knowledge of these proteins has grown over the years and aspects known and unknown about them. The current understanding suggests CBL-CIPKs to be a sensor-responder pair, where CBLs might ‘sense’ Calcium (Ca2+) and the signal response in effected through CIPKs. Surprisingly, CBLs were found to be homologous to one subunit of calcineurin and interact with a family of kinases, unlike calcineurin B, which interacts with a phosphatase. Now, two decades after their discovery, CBL-CIPKs have been characterized in a number of processes, especially in stress responses. The authors touch upon some hitherto unresolved aspects such as the elusive upstream activator of CIPKs, relevance of Ca2+ in CBL-CIPK-mediated responses, etc. Thus, this review article captures the shifting paradigm of CBL-CIPKs from their discovery, when, for example, CBLs were thought to interact exclusively with CIPKs, to now, when interaction of CBLs with other proteins has been conclusively demonstrated. (Summary by Pavithran Narayanan). Trends Plant Sci. 10.1016/j.tplants.2020.01.009 [altmetric doi=”10.1016/j.tplants.2020.01.009″ details=”right” float=”right”]
What are the chances that homologs of a vital animal protein could be found in plants, and how much structural and functional similarity would they share? These questions led to the discovery of Calcineurin B-like (CBL) and CBL-interacting protein kinase (CIPK) protein families in plants. In this review, Tang and co-workers discuss how knowledge of these proteins has grown over the years and aspects known and unknown about them. The current understanding suggests CBL-CIPKs to be a sensor-responder pair, where CBLs might ‘sense’ Calcium (Ca2+) and the signal response in effected through CIPKs. Surprisingly, CBLs were found to be homologous to one subunit of calcineurin and interact with a family of kinases, unlike calcineurin B, which interacts with a phosphatase. Now, two decades after their discovery, CBL-CIPKs have been characterized in a number of processes, especially in stress responses. The authors touch upon some hitherto unresolved aspects such as the elusive upstream activator of CIPKs, relevance of Ca2+ in CBL-CIPK-mediated responses, etc. Thus, this review article captures the shifting paradigm of CBL-CIPKs from their discovery, when, for example, CBLs were thought to interact exclusively with CIPKs, to now, when interaction of CBLs with other proteins has been conclusively demonstrated. (Summary by Pavithran Narayanan). Trends Plant Sci. 10.1016/j.tplants.2020.01.009 [altmetric doi=”10.1016/j.tplants.2020.01.009″ details=”right” float=”right”]