Review: Protein phosphorylation “toggle switch” for plant iron balance (Trends Plant Sci)
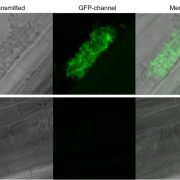
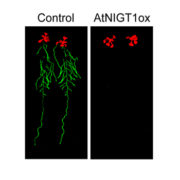
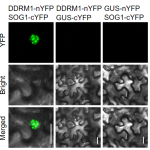
 Protein phosphorylation and dephosphorylation act as a switch regulating a multitude of protein properties, be it their activity, interaction with other proteins, stability, or even cellular localization. This review by Li et al. describs the many ways that protein phosphorylation contributes to iron uptake and homeostasis. The membrane localization of the iron transporter IRT1 is regulated by Sorting Nexin 1 (SNX1), and phosphorylation of SNX1and IRT1 regulates iron uptake and the intracellular iron pool. When exposed to above-optimal zinc and manganese concentrations, phosphorylation by the CBL-Interacting protein kinase 23 (CIPK23) targets IRT1 for proteasomal degradation and prevents IRT1 mediated non-Fe metal transport. Iron homeostasis regulators like the bHLH family transcription factors FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT) and POPEYE (PYE) are also regulated by phosphorylation. For example, phosphorylation of FIT at Ser272 by the CIPK11-CBL1/9 network renders it active while phosphorylation at Tyr 238 and Tyr 272, putatively by MAP kinases MPK3 and MPK6, shuts it down. The UPSTREAM REGULATOR OF IRT1 (URI), a modulator of FIT and PYE, is regulated by iron-status dependent phosphorylation for both activity and tissue localization. Under iron deficiency, URI is phosphorylated, and the formation of its heterodimer with bHLH TFs is promoted. The heterodimer formation of URI drives the transcription of key members of the bHLHIb TF family that are inducers of FIT dependent iron uptake responses. Finally, ethylene and nitric oxide-mediated iron deficiency responses are also immensely reliant on protein phosphorylation. Protein phosphorylation is thus an essential strategy for modulating key regulators to ensure a highly sensitive and precise response to iron availability. (Summary by Lekshmy Sathee @lekshmysnair) Trends Plant Sci. 10.1016/j.tplants.2022.03.012
Protein phosphorylation and dephosphorylation act as a switch regulating a multitude of protein properties, be it their activity, interaction with other proteins, stability, or even cellular localization. This review by Li et al. describs the many ways that protein phosphorylation contributes to iron uptake and homeostasis. The membrane localization of the iron transporter IRT1 is regulated by Sorting Nexin 1 (SNX1), and phosphorylation of SNX1and IRT1 regulates iron uptake and the intracellular iron pool. When exposed to above-optimal zinc and manganese concentrations, phosphorylation by the CBL-Interacting protein kinase 23 (CIPK23) targets IRT1 for proteasomal degradation and prevents IRT1 mediated non-Fe metal transport. Iron homeostasis regulators like the bHLH family transcription factors FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT) and POPEYE (PYE) are also regulated by phosphorylation. For example, phosphorylation of FIT at Ser272 by the CIPK11-CBL1/9 network renders it active while phosphorylation at Tyr 238 and Tyr 272, putatively by MAP kinases MPK3 and MPK6, shuts it down. The UPSTREAM REGULATOR OF IRT1 (URI), a modulator of FIT and PYE, is regulated by iron-status dependent phosphorylation for both activity and tissue localization. Under iron deficiency, URI is phosphorylated, and the formation of its heterodimer with bHLH TFs is promoted. The heterodimer formation of URI drives the transcription of key members of the bHLHIb TF family that are inducers of FIT dependent iron uptake responses. Finally, ethylene and nitric oxide-mediated iron deficiency responses are also immensely reliant on protein phosphorylation. Protein phosphorylation is thus an essential strategy for modulating key regulators to ensure a highly sensitive and precise response to iron availability. (Summary by Lekshmy Sathee @lekshmysnair) Trends Plant Sci. 10.1016/j.tplants.2022.03.012