Prion-like domains of sensory HSFs remember heat
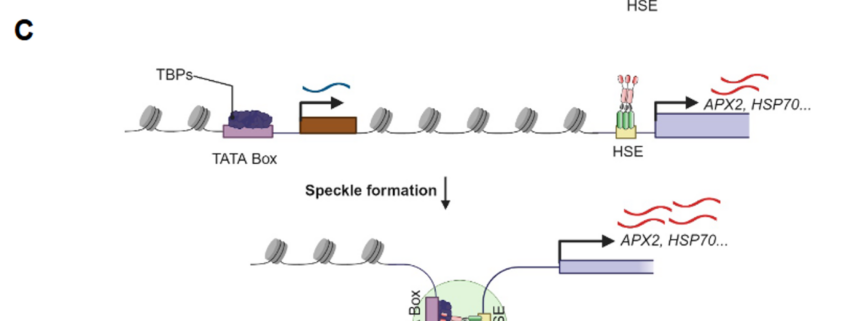
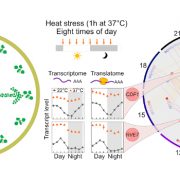
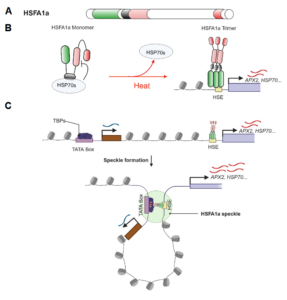 The heat shock response, a rapid transcriptional response to heat, was first observed nearly 60 years ago, and has long been a paradigm for understanding gene responses to exogenous cues. The family of genes encoding heat shock factors (HSFs) is greatly expanded in plants. These HSFs serve as key regulators of the heat shock response, both initiating short-term transcriptional changes, but also serving as a molecular memory that activates heat memory genes after an earlier “priming” stress. A new study by Peng et al. examined how conformational changes in HSF protein structure contributes to both short term and memory responses to heat shock. The authors identified a transcriptional cascade, led by rapid activation HSFA1 by protein conformational changes. Within moments of heat shock, HSFA1 factors form speckles in the nucleus, suggesting that they act as heat sensors, and eliciting a very rapid transcriptional response. HSFA1 factors contain two Prion-related Domains (PrDs), which the authors showed are critical for their thermal responsiveness. PrDs are intrinsically disordered domains that are often found in proteins that undergo liquid phase separation. One of these, PrD1, is responsible for sequestering the HSFA1 proteins during ambient temperatures and maintaining them in an inactive state. The other, PrD2, is required for heat activation and thermal memory, through the formation of DNA loops that connect heat-responsive promoters with enhancer domains. This work builds on the growing recognition of the importance of intrinsically disordered domains and liquid phase separation in modulating rapid cellular responses. (Summary by Mary Williams @PlantTeaching.bsky.social) Mol Plant 10.1016/j.molp.2025.01.007
The heat shock response, a rapid transcriptional response to heat, was first observed nearly 60 years ago, and has long been a paradigm for understanding gene responses to exogenous cues. The family of genes encoding heat shock factors (HSFs) is greatly expanded in plants. These HSFs serve as key regulators of the heat shock response, both initiating short-term transcriptional changes, but also serving as a molecular memory that activates heat memory genes after an earlier “priming” stress. A new study by Peng et al. examined how conformational changes in HSF protein structure contributes to both short term and memory responses to heat shock. The authors identified a transcriptional cascade, led by rapid activation HSFA1 by protein conformational changes. Within moments of heat shock, HSFA1 factors form speckles in the nucleus, suggesting that they act as heat sensors, and eliciting a very rapid transcriptional response. HSFA1 factors contain two Prion-related Domains (PrDs), which the authors showed are critical for their thermal responsiveness. PrDs are intrinsically disordered domains that are often found in proteins that undergo liquid phase separation. One of these, PrD1, is responsible for sequestering the HSFA1 proteins during ambient temperatures and maintaining them in an inactive state. The other, PrD2, is required for heat activation and thermal memory, through the formation of DNA loops that connect heat-responsive promoters with enhancer domains. This work builds on the growing recognition of the importance of intrinsically disordered domains and liquid phase separation in modulating rapid cellular responses. (Summary by Mary Williams @PlantTeaching.bsky.social) Mol Plant 10.1016/j.molp.2025.01.007