Plant Science Research Weekly: October 2, 2020
Review: Dry architecture: towards the understanding of the variation of longevity in desiccation- tolerant germplasm ($)
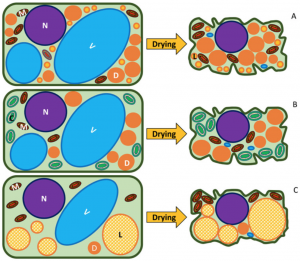 Most seeds, pollen grains, and fern spores are desiccation-tolerant, meaning they remain viable after drying to low water contents. Given this property, they can be stored in cold and dry conditions and potentially remain alive for several years. However, there is increasing evidence that seed longevity in storage conditions might be much shorter than initially thought. In this fascinating review, Ballesteros and colleagues provide an overview of the drying process of desiccation-tolerant germplasm and how the dry cytoplasm’s composition and structure might contribute to viability loss. As germplasm dries, cellular structures are compacted, and cytoplasm becomes a glassy matrix that restricts molecules’ free flow. However, the physical properties of this matrix can be shaped by the composition of the cytoplasm. For instance, chlorophyllous cells’ presence predisposes germplasm to age, given the production of free radicals by chlorophyll in response to light. Moreover, germplasm with high contents of storage lipids tends to age quickly, given that lipid crystallization destabilizes the cytoplasm matrix, allowing the free flow of metabolites throughout it. Given this, the authors highlight the importance of a deeper understanding of dry cytoplasm composition and structure to enhance the development of new germplasms banking technologies that optimizes germplasm longevity. (Summary by Carlos A. Ordóñez-Parra @caordonezparra) Seed Sci. Res. 10.1017/S0960258520000239
Most seeds, pollen grains, and fern spores are desiccation-tolerant, meaning they remain viable after drying to low water contents. Given this property, they can be stored in cold and dry conditions and potentially remain alive for several years. However, there is increasing evidence that seed longevity in storage conditions might be much shorter than initially thought. In this fascinating review, Ballesteros and colleagues provide an overview of the drying process of desiccation-tolerant germplasm and how the dry cytoplasm’s composition and structure might contribute to viability loss. As germplasm dries, cellular structures are compacted, and cytoplasm becomes a glassy matrix that restricts molecules’ free flow. However, the physical properties of this matrix can be shaped by the composition of the cytoplasm. For instance, chlorophyllous cells’ presence predisposes germplasm to age, given the production of free radicals by chlorophyll in response to light. Moreover, germplasm with high contents of storage lipids tends to age quickly, given that lipid crystallization destabilizes the cytoplasm matrix, allowing the free flow of metabolites throughout it. Given this, the authors highlight the importance of a deeper understanding of dry cytoplasm composition and structure to enhance the development of new germplasms banking technologies that optimizes germplasm longevity. (Summary by Carlos A. Ordóñez-Parra @caordonezparra) Seed Sci. Res. 10.1017/S0960258520000239
Review: Biomolecular condensates in photosynthesis and metabolism ($)
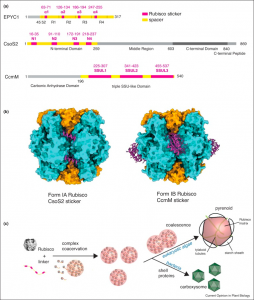 Biomolecular condensates are membraneless organelles with the capacity to spatially concentrate biomolecules. Liquid-liquid phase separation (LLPS) is one mechanism of condensate formation in which demixing of macromolecules leads to separation into dense and light phases. Photosynthetic organisms like cyanobacteria and green algae also form CO2-fixing compartments as biomolecular condensates through LLPS. In this paper, Wunder et al. review the role of LLPS in the formation of Rubisco-containing condensates. The authors highlight the formation of Rubisco complexes like carboxysomes in proteobacteria and pyrenoids in microalgae, and their role in increasing the enzyme efficiency and specificity of CO2 assimilation. In addition, the authors discuss how the highly rigid cuboid structure of Rubisco complexes favors multivalent interactions for condensate formation. The authors also review the structure of three Rubisco-linking scaffold proteins, their stickers and spacer regions and their role in condensate formation. Overall, the authors provide an insight to the importance of condensates in metabolic channeling and focus on enhancing knowledge on the spatiotemporal regulation of condensate formation for future use in synthetic biology. (Summary by Sunita Pathak @psunita980) Curr. Opin. Plant Biol. 10.1016/j.pbi.2020.08.006
Biomolecular condensates are membraneless organelles with the capacity to spatially concentrate biomolecules. Liquid-liquid phase separation (LLPS) is one mechanism of condensate formation in which demixing of macromolecules leads to separation into dense and light phases. Photosynthetic organisms like cyanobacteria and green algae also form CO2-fixing compartments as biomolecular condensates through LLPS. In this paper, Wunder et al. review the role of LLPS in the formation of Rubisco-containing condensates. The authors highlight the formation of Rubisco complexes like carboxysomes in proteobacteria and pyrenoids in microalgae, and their role in increasing the enzyme efficiency and specificity of CO2 assimilation. In addition, the authors discuss how the highly rigid cuboid structure of Rubisco complexes favors multivalent interactions for condensate formation. The authors also review the structure of three Rubisco-linking scaffold proteins, their stickers and spacer regions and their role in condensate formation. Overall, the authors provide an insight to the importance of condensates in metabolic channeling and focus on enhancing knowledge on the spatiotemporal regulation of condensate formation for future use in synthetic biology. (Summary by Sunita Pathak @psunita980) Curr. Opin. Plant Biol. 10.1016/j.pbi.2020.08.006
Small subunits can determine enzyme kinetics of tobacco Rubisco expressed in Escherichia coli
 Rubisco is the enzyme responsible for the fixation of CO2 to ribulose-1,5-bisphosphate (RuBP) during photosynthetic reactions. However, this enzyme has some functional issues, such as a slow catalytic turnover rate and sensitivity to temperature and CO2, and it catalyzes a competing oxygenation process in C3 plants. Crops have reached a photosynthetic yield plateau that could be enhanced with a more efficient carbon fixation process. Plants have form-I Rubisco, with 8 large (RbcLs) and 8 small subunits (RbcSs). RbcLs tend to easily aggregate and depend on chaperones for proper folding and dimerization, impairing heterologous expression in bacteria for functional studies. Lin et al. modified and improved methods previously described to coexpress Rubisco with chaperonin and chaperones from Arabidopsis and tobacco using T7 promoters under autoinduction conditions. Eight different tobacco RbcSs were coexpressed with RbcL and assayed for proper assembly and kinetics efficiency. Rubisco assembled in E. coli from tobacco trichome RbcS-Tc showed higher turnover but a lower affinity for CO2 than native mesophyll RbcSs, as previously described for Rubisco found in trichomes. These results demonstrate that RbcSs can determine tobacco Rubisco kinetics when expressed in E. coli. The described strategies can be further used to screen and engineer Rubisco with improved function from other crop plants. (Summary by Camila Ribeiro @camicribeiro86) Nature Plants 10.1038/s
Rubisco is the enzyme responsible for the fixation of CO2 to ribulose-1,5-bisphosphate (RuBP) during photosynthetic reactions. However, this enzyme has some functional issues, such as a slow catalytic turnover rate and sensitivity to temperature and CO2, and it catalyzes a competing oxygenation process in C3 plants. Crops have reached a photosynthetic yield plateau that could be enhanced with a more efficient carbon fixation process. Plants have form-I Rubisco, with 8 large (RbcLs) and 8 small subunits (RbcSs). RbcLs tend to easily aggregate and depend on chaperones for proper folding and dimerization, impairing heterologous expression in bacteria for functional studies. Lin et al. modified and improved methods previously described to coexpress Rubisco with chaperonin and chaperones from Arabidopsis and tobacco using T7 promoters under autoinduction conditions. Eight different tobacco RbcSs were coexpressed with RbcL and assayed for proper assembly and kinetics efficiency. Rubisco assembled in E. coli from tobacco trichome RbcS-Tc showed higher turnover but a lower affinity for CO2 than native mesophyll RbcSs, as previously described for Rubisco found in trichomes. These results demonstrate that RbcSs can determine tobacco Rubisco kinetics when expressed in E. coli. The described strategies can be further used to screen and engineer Rubisco with improved function from other crop plants. (Summary by Camila Ribeiro @camicribeiro86) Nature Plants 10.1038/s
Roles for this ROP GTPase in subcellular and tissue-level patterning
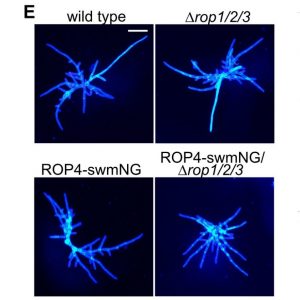 Rho-of-plants (ROP) proteins are master regulators of cell polarity within plants and influence cell wall deposition, tissue development, and several signaling processes. However, they are quite difficult to study: attempts to form a fluorescent ROP fusion protein have proven elusive, and there are multiple functionally redundant ROP family members in seed plants, whereas Physcomitrium patens contains only four ROP proteins. As ROPs are promiscuous and contain multiple interaction sites, choosing the “wrong” site to tag can (and does, as the authors of this paper show) negatively affect native protein function, generating inaccurate data. In this study, Cheng et al. used P. patens to create a mutant line containing null mutations of ROP1/2/3 and an edited ROP4 with a mNeonGreen “sandwich” tag in the middle of the sequence, generating a fluorescently-tagged ROP4 (referred to by the authors and further in this summary as “ROP4-swmNG”) that did not interfere with known interaction sites; was phenotypically similar to the WT; and sufficiently rescued ROP activity. Using this powerful tool, the authors were able to determine notable ROP4 functions. During protonemata development, ROP4-swmNG accumulated at the apical tip of actively growing cells, and their accumulation was shown to consistently predict the sites of cellular expansion. Further investigations suggested that the localization of ROP4-swmNG was maintained by both actin and microtubules, but specifically in the early stages of the cell cycle, hinting at a cell cycle function. Such studies are vital towards both inspirational tool development and further characterization of important plant growth components. (Summary by Benjamin Jin) Plant Cell 10.1105/tpc.20.00440
Rho-of-plants (ROP) proteins are master regulators of cell polarity within plants and influence cell wall deposition, tissue development, and several signaling processes. However, they are quite difficult to study: attempts to form a fluorescent ROP fusion protein have proven elusive, and there are multiple functionally redundant ROP family members in seed plants, whereas Physcomitrium patens contains only four ROP proteins. As ROPs are promiscuous and contain multiple interaction sites, choosing the “wrong” site to tag can (and does, as the authors of this paper show) negatively affect native protein function, generating inaccurate data. In this study, Cheng et al. used P. patens to create a mutant line containing null mutations of ROP1/2/3 and an edited ROP4 with a mNeonGreen “sandwich” tag in the middle of the sequence, generating a fluorescently-tagged ROP4 (referred to by the authors and further in this summary as “ROP4-swmNG”) that did not interfere with known interaction sites; was phenotypically similar to the WT; and sufficiently rescued ROP activity. Using this powerful tool, the authors were able to determine notable ROP4 functions. During protonemata development, ROP4-swmNG accumulated at the apical tip of actively growing cells, and their accumulation was shown to consistently predict the sites of cellular expansion. Further investigations suggested that the localization of ROP4-swmNG was maintained by both actin and microtubules, but specifically in the early stages of the cell cycle, hinting at a cell cycle function. Such studies are vital towards both inspirational tool development and further characterization of important plant growth components. (Summary by Benjamin Jin) Plant Cell 10.1105/tpc.20.00440
Arabinogalactan-proteins of Zostera marina L. contain unique glycan structures and provide insight into adaption processes to saline environments
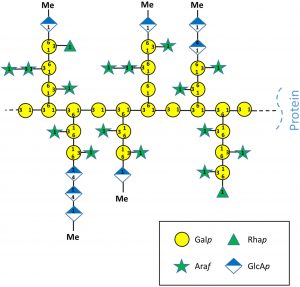 Seagrasses are fascinating examples of adaptation. Like dolphins and other sea mammals, they are marine-dwelling descendants of species that had fully adapted to life on land. To survive submerged in saltwater has necessitated morphological, anatomical and physiological adaptations. Recent genome analysis suggests that seagrasses also have unique cell wall components. Pfeifer et al. have used several proteomic and analytical methods to investigate the arabinogalactan proteins (AGPs; highly glycosylated cell-surface proteins), of the seagrass Zostera marina. AGPs in terrestrial angiosperms are known to play roles in cellular signaling as well as in salt responses. The authors found that the seagrass AGPs include many non-canonical sequences and have unique polysaccharide moieties. Specifically, these polysaccharides are highly branched and have a high Araf (α-L-arabinose) content; arabinose has previously been shown to contribute to salt stress tolerance in Arabidopsis and to contribute plasticity to walls of resurrection plants. They also are enriched for uronic acids which are known to facilitate calcium binding. These findings suggest that the adaptations of seagrasses to saline waters involves extensive cell wall modifications. (Summary by Mary Williams @PlantTeaching) Sci. Reports 10.1038/s41598-020-65135-5
Seagrasses are fascinating examples of adaptation. Like dolphins and other sea mammals, they are marine-dwelling descendants of species that had fully adapted to life on land. To survive submerged in saltwater has necessitated morphological, anatomical and physiological adaptations. Recent genome analysis suggests that seagrasses also have unique cell wall components. Pfeifer et al. have used several proteomic and analytical methods to investigate the arabinogalactan proteins (AGPs; highly glycosylated cell-surface proteins), of the seagrass Zostera marina. AGPs in terrestrial angiosperms are known to play roles in cellular signaling as well as in salt responses. The authors found that the seagrass AGPs include many non-canonical sequences and have unique polysaccharide moieties. Specifically, these polysaccharides are highly branched and have a high Araf (α-L-arabinose) content; arabinose has previously been shown to contribute to salt stress tolerance in Arabidopsis and to contribute plasticity to walls of resurrection plants. They also are enriched for uronic acids which are known to facilitate calcium binding. These findings suggest that the adaptations of seagrasses to saline waters involves extensive cell wall modifications. (Summary by Mary Williams @PlantTeaching) Sci. Reports 10.1038/s41598-020-65135-5
Origins and evolution of cuticle biosynthetic machinery in land plants
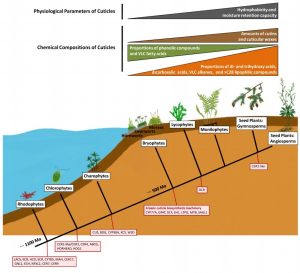 The availability of annotated genomes for a wide range of plants allows for tremendous opportunity to perform comparative genomics studies such as that carried out by Kong et al. detailing the evolution of cuticle biosynthesis. The cuticle is the waxy hydrophobic layer that protects aerial plant tissue from dehydration and environmental stresses. Its synthesis involves several components, hypothesized to have evolved as a means for plants to adapt to land colonization. The authors identified the genomic components of cuticle biosynthesis within 41 different plant species covering the major lineages, from algal species to several angiosperms including the model organism Arabidopsis thaliana. While algal species only contain partial sets of cuticle biosynthesis genes, bryophytes comprise a basic set of cuticle biosynthesis machinery, and spermatophytes, the seed plants, express the most robust set of cuticle biosynthesis genes. This genomics work was coupled with chemical composition and phenotype analysis of cuticles from 13 representative plant species. Notably, cutin and wax loads in cuticles of seed plants were significantly higher in mass proportion measurements than in those of monilophytes, lycophytes, and bryophytes, with bryophytes having the lowest proportions of all. Biochemical composition of cuticular components from bryophytic to spermatophytic species changed as well. Further, spermatophyte cuticles demonstrated greater hydrophobicity and moisture retention ability than other plant species. This study confirms that components of cuticle biosynthesis machinery first evolved in algal species, but “canonical” cuticles originated in the first land plant ancestors and developed into a key adaptation for terrestrial colonization. (Summary by Benjamin Jin) Plant Physiology 10.1104/pp.20.00913
The availability of annotated genomes for a wide range of plants allows for tremendous opportunity to perform comparative genomics studies such as that carried out by Kong et al. detailing the evolution of cuticle biosynthesis. The cuticle is the waxy hydrophobic layer that protects aerial plant tissue from dehydration and environmental stresses. Its synthesis involves several components, hypothesized to have evolved as a means for plants to adapt to land colonization. The authors identified the genomic components of cuticle biosynthesis within 41 different plant species covering the major lineages, from algal species to several angiosperms including the model organism Arabidopsis thaliana. While algal species only contain partial sets of cuticle biosynthesis genes, bryophytes comprise a basic set of cuticle biosynthesis machinery, and spermatophytes, the seed plants, express the most robust set of cuticle biosynthesis genes. This genomics work was coupled with chemical composition and phenotype analysis of cuticles from 13 representative plant species. Notably, cutin and wax loads in cuticles of seed plants were significantly higher in mass proportion measurements than in those of monilophytes, lycophytes, and bryophytes, with bryophytes having the lowest proportions of all. Biochemical composition of cuticular components from bryophytic to spermatophytic species changed as well. Further, spermatophyte cuticles demonstrated greater hydrophobicity and moisture retention ability than other plant species. This study confirms that components of cuticle biosynthesis machinery first evolved in algal species, but “canonical” cuticles originated in the first land plant ancestors and developed into a key adaptation for terrestrial colonization. (Summary by Benjamin Jin) Plant Physiology 10.1104/pp.20.00913
Vascular transcription factors guide plant epidermal responses to limiting phosphate conditions
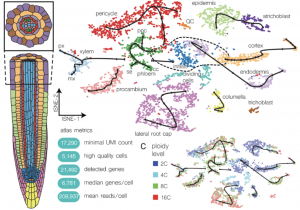 Plants produce more root hairs (epidermal projections) in response to low soil phosphate and the detailed mechanism of this developmental response remains elusive. TARGET OF MONOPTEROS 5 (TMO5) and LONESOME HIGHWAY (LHW) are vascular specific bHLH proteins that work as a heterodimer to activate the rate limiting enzyme LONELY GUY4 (LOG4) in cytokinin biosynthesis. Based on the available literature it is difficult to envision any connection between these vascular specific TMO5/LHW and epidermal cell morphology. In this paper, Wendrich, Yang, Vandamme, Verstaen et al., identified a cell non-autonomous function of TMO5/LHW in root hair cell formation under low phosphate conditions. The authors used 10X genomics single cell RNA-Seq in Arabidopsis roots to generate a high-resolution gene expression atlas and identified that 47 target genes of TMO5/LHW are exclusively expressed in roots hairs. Single and double mutants of TMO5 and TMO5-LIKE1 do not show any abnormal root hair phenotype under normal conditions but are required for root hair formation under low phosphate conditions. Under low phosphate conditions TMO5 expression increases in the root vasculature producing more cytokinin which diffuses to the epidermal cells to activate TMO5 target genes. Further, vascular-specific increase or decrease of cytokinin signaling shows increased or decreased root hair phenotype respectively. Thus, this paper identifies the mechanism by which vascular-specific transcription factors activates cytokinin as a mediator to induce root hairs in cell non-autonomous manner. Summary by (Vijaya Batthula @Vijaya_Batthula) Science 10.1126/science.aay4970
Plants produce more root hairs (epidermal projections) in response to low soil phosphate and the detailed mechanism of this developmental response remains elusive. TARGET OF MONOPTEROS 5 (TMO5) and LONESOME HIGHWAY (LHW) are vascular specific bHLH proteins that work as a heterodimer to activate the rate limiting enzyme LONELY GUY4 (LOG4) in cytokinin biosynthesis. Based on the available literature it is difficult to envision any connection between these vascular specific TMO5/LHW and epidermal cell morphology. In this paper, Wendrich, Yang, Vandamme, Verstaen et al., identified a cell non-autonomous function of TMO5/LHW in root hair cell formation under low phosphate conditions. The authors used 10X genomics single cell RNA-Seq in Arabidopsis roots to generate a high-resolution gene expression atlas and identified that 47 target genes of TMO5/LHW are exclusively expressed in roots hairs. Single and double mutants of TMO5 and TMO5-LIKE1 do not show any abnormal root hair phenotype under normal conditions but are required for root hair formation under low phosphate conditions. Under low phosphate conditions TMO5 expression increases in the root vasculature producing more cytokinin which diffuses to the epidermal cells to activate TMO5 target genes. Further, vascular-specific increase or decrease of cytokinin signaling shows increased or decreased root hair phenotype respectively. Thus, this paper identifies the mechanism by which vascular-specific transcription factors activates cytokinin as a mediator to induce root hairs in cell non-autonomous manner. Summary by (Vijaya Batthula @Vijaya_Batthula) Science 10.1126/science.aay4970