Plant Science Research Weekly: January 29, 2021
Review: Selective redox signaling shapes plant-pathogen interactions
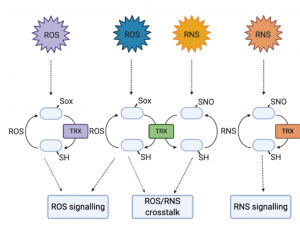 Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are often presented as something of enigmas. They are damaging by-products of metabolism and stress, but also intentionally produced as a signal and defense response to pathogens. This excellent Update by Bleau and Spoel synthesizes new work towards understanding these apparently conflicting roles for ROS/RNS. First they describe the locations and fine regulation of ROS/RNS-producing enzymes (from plants and pathogens), and how these different sources contribute to responses. They then describe how the ROS/RNS function as signals, such as their selective post-translational modifications of proteins including the key defense protein and redox sensor NPR1, and discuss thioredoxins that are emerging as central players in selective redox signaling. The authors also describe the new proteomics methods that have been instrumental in these perspectives. Finally, the discuss how the pathogens have escalated the arms race by “hijacking and manipulating” the plant’s redox-dependent immune response. (Summary by Mary Williams @PlantTeaching) Plant Physiol. 10.1093/plphys/kiaa088/6117245
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are often presented as something of enigmas. They are damaging by-products of metabolism and stress, but also intentionally produced as a signal and defense response to pathogens. This excellent Update by Bleau and Spoel synthesizes new work towards understanding these apparently conflicting roles for ROS/RNS. First they describe the locations and fine regulation of ROS/RNS-producing enzymes (from plants and pathogens), and how these different sources contribute to responses. They then describe how the ROS/RNS function as signals, such as their selective post-translational modifications of proteins including the key defense protein and redox sensor NPR1, and discuss thioredoxins that are emerging as central players in selective redox signaling. The authors also describe the new proteomics methods that have been instrumental in these perspectives. Finally, the discuss how the pathogens have escalated the arms race by “hijacking and manipulating” the plant’s redox-dependent immune response. (Summary by Mary Williams @PlantTeaching) Plant Physiol. 10.1093/plphys/kiaa088/6117245
Review. The Fast and The Furious: Rapid long-range signaling in plants
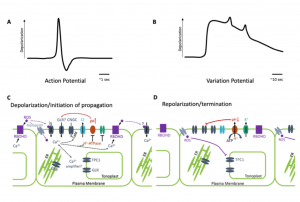 Plants possess a complex series of local and systemic signaling networks driven by multiple stimuli. They translate this information into plant-wide response to coordinate their physiology and development. Here, Johns et al. review these communication pathways, which function across different scales and timeframes. Mobile chemical signals such as auxin, RNAs, peptides and small molecules move from cell-to-cell at a slow and steady speed. More rapid systemic signaling, such as triggers leaf movements in mimosa or Venus fly traps, is thought to be associated with electrical changes. In this rapid signalling, mechanical stimulation of the sensory hair generates a transient depolarization of the membrane potential, known as action potential (AP). Other stimuli such as wounding are associated with a slow wave potential called a variation potential (VP). Much of the focus of this review is on the membrane-localized channels, pumps and transporters that contribute to these electric and ionic signals, and also the contributions of calcium, glutamate and reactive oxygen in these rapid signals. (Summary by Min May Wong @wongminmay) Plant Physiol. https://doi.org/10.1093/plphys/kiaa098
Plants possess a complex series of local and systemic signaling networks driven by multiple stimuli. They translate this information into plant-wide response to coordinate their physiology and development. Here, Johns et al. review these communication pathways, which function across different scales and timeframes. Mobile chemical signals such as auxin, RNAs, peptides and small molecules move from cell-to-cell at a slow and steady speed. More rapid systemic signaling, such as triggers leaf movements in mimosa or Venus fly traps, is thought to be associated with electrical changes. In this rapid signalling, mechanical stimulation of the sensory hair generates a transient depolarization of the membrane potential, known as action potential (AP). Other stimuli such as wounding are associated with a slow wave potential called a variation potential (VP). Much of the focus of this review is on the membrane-localized channels, pumps and transporters that contribute to these electric and ionic signals, and also the contributions of calcium, glutamate and reactive oxygen in these rapid signals. (Summary by Min May Wong @wongminmay) Plant Physiol. https://doi.org/10.1093/plphys/kiaa098
In vivo single-particle tracking of the aquaporin AtPIP2;1 in stomata reveals cell type-specific dynamics
 Bacteria can exploit the stomatal pores to gain entry into plant leaves. Previous studies have demonstrated that guard cells close in response to flagellin, an effect that is described as stomatal immunity. Like ABA-induced stomatal closure, this involves the movements of ions and water from the guard cells. Here, Cui et al. investigated the contribution of the water channel aquaporin PIP2;1 in stomatal immunity, as previous studies indicated that loss-of-function pip2;1 mutants fail to respond to ABA or the flagellin-epitope flg22. In wild-type and mutant tissues, the authors measured ionic and pH responses to flg22, as well as PIP2;1 localization in guard cells and subsidiary cells using variable-angle total internal reflection fluorescence microscopy (VA-TIRFM), which allowed them to follow the movements of single particles. Key findings are that the aquaporin moves into the cell from the plasma membrane surface during flg22-mediated stomatal closure, and that the cytoskeleton contributes to PIP2;1 dynamics. (Summary by Mary Williams @PlantTeaching) Plant Physiol. 10.1093/plphys/kiab007/6094659
Bacteria can exploit the stomatal pores to gain entry into plant leaves. Previous studies have demonstrated that guard cells close in response to flagellin, an effect that is described as stomatal immunity. Like ABA-induced stomatal closure, this involves the movements of ions and water from the guard cells. Here, Cui et al. investigated the contribution of the water channel aquaporin PIP2;1 in stomatal immunity, as previous studies indicated that loss-of-function pip2;1 mutants fail to respond to ABA or the flagellin-epitope flg22. In wild-type and mutant tissues, the authors measured ionic and pH responses to flg22, as well as PIP2;1 localization in guard cells and subsidiary cells using variable-angle total internal reflection fluorescence microscopy (VA-TIRFM), which allowed them to follow the movements of single particles. Key findings are that the aquaporin moves into the cell from the plasma membrane surface during flg22-mediated stomatal closure, and that the cytoskeleton contributes to PIP2;1 dynamics. (Summary by Mary Williams @PlantTeaching) Plant Physiol. 10.1093/plphys/kiab007/6094659
Deeply altered genome architecture in the endoparasitic flowering plant Sapria himalayana Griff. (Rafflesiaceae)
 It’s one of those plants that gets noticed, so we’re excited to see the press associated with the genome sequence of the large endoparasitic plant Sapria himalayana. Everyone has seen pictures of this plant, which lives entirely underground except when it produces its large (20 cm diameter) blood red flowers. Cai et al. describe how this weird lifestyle is reflected in a weird genome. Like other parasitic plants, it has lost many otherwise conserved genes, but in this case fully 44% of conserved plant genes are missing, including apparently all plastid and plastid-associated genes. It also notably has some distinctly long introns, as long as 100 kb, potentially as a consequence of relaxed selective pressure. Finally, there is evidence of intimate associations with not only current, but also former hosts, in the form of horizontal gene transfer events. (Summary by Mary Williams @PlantTeaching) Curr. Biol. 10.1016/j.cub.2020.12.045
It’s one of those plants that gets noticed, so we’re excited to see the press associated with the genome sequence of the large endoparasitic plant Sapria himalayana. Everyone has seen pictures of this plant, which lives entirely underground except when it produces its large (20 cm diameter) blood red flowers. Cai et al. describe how this weird lifestyle is reflected in a weird genome. Like other parasitic plants, it has lost many otherwise conserved genes, but in this case fully 44% of conserved plant genes are missing, including apparently all plastid and plastid-associated genes. It also notably has some distinctly long introns, as long as 100 kb, potentially as a consequence of relaxed selective pressure. Finally, there is evidence of intimate associations with not only current, but also former hosts, in the form of horizontal gene transfer events. (Summary by Mary Williams @PlantTeaching) Curr. Biol. 10.1016/j.cub.2020.12.045
Seed tolerance to post-fire temperature fluctuation of Cerrado legume shrubs with micromorphological implications ($)
 Legume seeds are typically physically dormant, meaning they have an impermeable seed coat that prevents water uptake. In fire-prone ecosystems, this dormancy is presumably broken when the seed coat is cracked due to being exposed to fire or the daily temperature fluctuations found in burnt sites. However, the role of alternate temperatures in this process has been seldom addressed in tropical systems. Here, Daibes and colleagues test whether simulated post-fire temperature fluctuations affect the germination, viability, and seed coat micromorphology of nine Fabaceae species from the Brazilian Cerrado. Before germination trials, seeds were incubated under room temperature or post-fire temperature regimes (ranging from 18 to 55ºC). The seeds’ hilar region was blocked to test its relative importance for imbibition. Multiple germination responses were found, with fluctuating temperatures either reducing germination and viability or having no effect at all. For instance, scanning electron micrography showed that most species’ seed coat remained practically intact after exposure to post-fire temperature regimes. In contrast to evidence from other regions, this research casts doubt on the role of alternate temperature on breaking physical dormancy in this Neotropical ecosystem. (Summary by Carlos A. Ordóñez-Parra @caordonezparra) Flora 10.1016/j.flora.2020.151761
Legume seeds are typically physically dormant, meaning they have an impermeable seed coat that prevents water uptake. In fire-prone ecosystems, this dormancy is presumably broken when the seed coat is cracked due to being exposed to fire or the daily temperature fluctuations found in burnt sites. However, the role of alternate temperatures in this process has been seldom addressed in tropical systems. Here, Daibes and colleagues test whether simulated post-fire temperature fluctuations affect the germination, viability, and seed coat micromorphology of nine Fabaceae species from the Brazilian Cerrado. Before germination trials, seeds were incubated under room temperature or post-fire temperature regimes (ranging from 18 to 55ºC). The seeds’ hilar region was blocked to test its relative importance for imbibition. Multiple germination responses were found, with fluctuating temperatures either reducing germination and viability or having no effect at all. For instance, scanning electron micrography showed that most species’ seed coat remained practically intact after exposure to post-fire temperature regimes. In contrast to evidence from other regions, this research casts doubt on the role of alternate temperature on breaking physical dormancy in this Neotropical ecosystem. (Summary by Carlos A. Ordóñez-Parra @caordonezparra) Flora 10.1016/j.flora.2020.151761



