Plant Science Research Weekly: February 21, 2025
Perspective: How should the advancement of large language models affect the practice of science?
 In this thought-provoking Perspective, four sets of authors express their opinions about the use of Large Language Models (such as ChatGPT) in the practice of science. Each essay is well reasoned, and each identifies both strengths and limitations of LLMs. There’s agreement that LLMs are good for some things, such as transcribing audio recordings and searching for related manuscripts. However, these applications are vastly different than some of those proposed for LLMs, such as peer review of research publications, or anticipating how humans might respond to a survey. I encourage you to read this article and discuss it with your students or peers, as it is clearly something we all must be informed about. Personally, I fear that widespread use of LLMs will negatively impact the development of critical reading and writing skills of trainees, but I also worry about the perpetuation of inaccuracies as the products of LLMs feed forward into other LLM-crafted texts. Finally, the broad-scale elimination of scientific jobs occurring presently is in part being justified by the ability of LLMs and other AI approaches to “replace” human intellect, an idea that is morally and intellectually repellant. (Summary by Mary Williams @PlantTeaching.bsky.social) PNAS 10.1073/pnas.2401227121
In this thought-provoking Perspective, four sets of authors express their opinions about the use of Large Language Models (such as ChatGPT) in the practice of science. Each essay is well reasoned, and each identifies both strengths and limitations of LLMs. There’s agreement that LLMs are good for some things, such as transcribing audio recordings and searching for related manuscripts. However, these applications are vastly different than some of those proposed for LLMs, such as peer review of research publications, or anticipating how humans might respond to a survey. I encourage you to read this article and discuss it with your students or peers, as it is clearly something we all must be informed about. Personally, I fear that widespread use of LLMs will negatively impact the development of critical reading and writing skills of trainees, but I also worry about the perpetuation of inaccuracies as the products of LLMs feed forward into other LLM-crafted texts. Finally, the broad-scale elimination of scientific jobs occurring presently is in part being justified by the ability of LLMs and other AI approaches to “replace” human intellect, an idea that is morally and intellectually repellant. (Summary by Mary Williams @PlantTeaching.bsky.social) PNAS 10.1073/pnas.2401227121
Apoplastic pH acts as a chemical switch in plants by modulating H2O2 redox potential
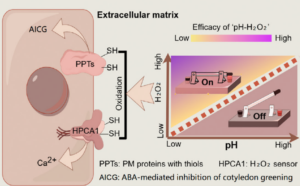 Environmental stimuli, such as drought and salinity, alter cellular conditions including apoplastic pH (pHApo) in plants. These stimuli often lead to an elevation in pHApo which is closely associated with cell functions and plant growth. However, the mechanism behind is largely unexplored. A recent study suggests that pHApo acts as a chemical switch, regulating signalling and modulating stress responses. Using Arabidopsis as the model, Zhou et al. tested the effect of abscisic acid (ABA) on cotyledon greening inhibition under different pHApo. ABA inhibits cotyledon greening by inducing apoplastic H2O2 production. Disrupting apoplastic H2O2 production alleviates the inhibition, and the elevation of pHApo mimics this effect. One might assume that pHApo elevation inhibits apoplastic H2O2 production, but the study reveals a different mechanism: instead of affecting H2O2 levels, increased pHApo lowers the redox potential. Since H2O2 oxidizes cysteine thiols of plasma membrane proteins to transduce stress signals, the lowered redox potential weakens this signal transduction, ultimately leading to stress alleviation. Using the plasma membrane-anchoring calcium signal regulator HPCA1 as an example, the authors showed that elevated pHApo weakened the H2O2-induced oxidation of HPCA1, thereby diminishing calcium signalling, and decreasing the plant sensitivity to H2O2 treatment. The study provides mechanistic insights into pHApo-mediated stress alleviation and highlights the potential to modulate stress responses by manipulating the chemical properties of signalling molecules. (Summary by Yee-Shan Ku @YeeShanKu1) New Phytol. 10.1111/nph.20400
Environmental stimuli, such as drought and salinity, alter cellular conditions including apoplastic pH (pHApo) in plants. These stimuli often lead to an elevation in pHApo which is closely associated with cell functions and plant growth. However, the mechanism behind is largely unexplored. A recent study suggests that pHApo acts as a chemical switch, regulating signalling and modulating stress responses. Using Arabidopsis as the model, Zhou et al. tested the effect of abscisic acid (ABA) on cotyledon greening inhibition under different pHApo. ABA inhibits cotyledon greening by inducing apoplastic H2O2 production. Disrupting apoplastic H2O2 production alleviates the inhibition, and the elevation of pHApo mimics this effect. One might assume that pHApo elevation inhibits apoplastic H2O2 production, but the study reveals a different mechanism: instead of affecting H2O2 levels, increased pHApo lowers the redox potential. Since H2O2 oxidizes cysteine thiols of plasma membrane proteins to transduce stress signals, the lowered redox potential weakens this signal transduction, ultimately leading to stress alleviation. Using the plasma membrane-anchoring calcium signal regulator HPCA1 as an example, the authors showed that elevated pHApo weakened the H2O2-induced oxidation of HPCA1, thereby diminishing calcium signalling, and decreasing the plant sensitivity to H2O2 treatment. The study provides mechanistic insights into pHApo-mediated stress alleviation and highlights the potential to modulate stress responses by manipulating the chemical properties of signalling molecules. (Summary by Yee-Shan Ku @YeeShanKu1) New Phytol. 10.1111/nph.20400
Diversity and genetic basis of hydropatterning in inbred maize lines
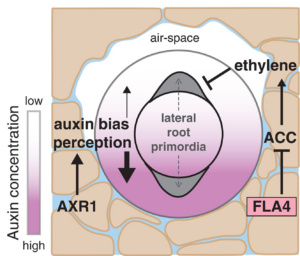 For more than a hundred years, plant biologists have been fascinated by how plants sense and respond to environments that are spatially and temporally heterogenous. Many of these responses occur through the remarkable developmental plasticity of plant growth, such as phototropism and photomorphogenesis. Below ground, root growth and branching patterns as well as root hair formation are highly tuned to the availability of water, nutrients, and even oxygen in the soil matrix. Hydropatterning is the process by which roots respond to water availability through changes in lateral root production. Studies of hydropatterning have been carried out in Arabidopsis, but in this new work, Scharwies et al. determined the extent of hydropatterning in 250 inbred maize lines. The authors found that the percentage of lateral roots forming on the dry size of the root ranged from 0 to 39%; higher proportions of dry-side lateral roots indicates weaker hydropatterning. Interestingly, the inbred lines with stronger hydropatterning also showed deeper roots systems with more brace roots, suggesting that the more efficient placement of lateral roots might allow root resources to be used more efficiently. The authors used GWAS and TWAS approaches to investigate the genetic controls underpinning hydropatterning, and identified several genes involved in auxin signaling, similar to what has been observed in Arabidopsis, as well as genes involved in ethylene synthesis and signaling. The authors also identified a role for an fasciclin-like arabinogalactan protein in hydropatterning, potentially through sensing of environmental cues. (Summary by Mary Williams @PlantTeaching.bsky.social) Science 10.1126/science.ads5999
For more than a hundred years, plant biologists have been fascinated by how plants sense and respond to environments that are spatially and temporally heterogenous. Many of these responses occur through the remarkable developmental plasticity of plant growth, such as phototropism and photomorphogenesis. Below ground, root growth and branching patterns as well as root hair formation are highly tuned to the availability of water, nutrients, and even oxygen in the soil matrix. Hydropatterning is the process by which roots respond to water availability through changes in lateral root production. Studies of hydropatterning have been carried out in Arabidopsis, but in this new work, Scharwies et al. determined the extent of hydropatterning in 250 inbred maize lines. The authors found that the percentage of lateral roots forming on the dry size of the root ranged from 0 to 39%; higher proportions of dry-side lateral roots indicates weaker hydropatterning. Interestingly, the inbred lines with stronger hydropatterning also showed deeper roots systems with more brace roots, suggesting that the more efficient placement of lateral roots might allow root resources to be used more efficiently. The authors used GWAS and TWAS approaches to investigate the genetic controls underpinning hydropatterning, and identified several genes involved in auxin signaling, similar to what has been observed in Arabidopsis, as well as genes involved in ethylene synthesis and signaling. The authors also identified a role for an fasciclin-like arabinogalactan protein in hydropatterning, potentially through sensing of environmental cues. (Summary by Mary Williams @PlantTeaching.bsky.social) Science 10.1126/science.ads5999
Healing Plants: how bacterial cellulose boosts plant regeneration
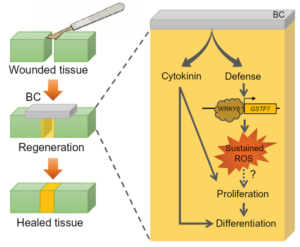 Have you ever placed a leaf from a Monstera plant in a glass of water and watched it grow new roots, forming a new plant? This happens because plants have a much greater ability to regenerate their tissues compared to animals. Recently, in Ruiz-Solaní et al., Spanish researchers have found a way to improve this regeneration, applying bacterial cellulose (BC) to plant wounds. BC is a biopolymer produced by specific bacterial strains that has been widely utilized in human biomedical applications as a scaffold for tissue regeneration. They found that applying bacterial cellulose to artificially cut leaves improved tissue regeneration—in other words, the cuts healed. Additionally, the compound contains plant hormones, such as cytokinin and auxins, which promote cell proliferation and differentiation. Additionally, BC activates plant defense mechanisms against microbial threats, particularly by inducing a reactive oxygen species (ROS) burst, a key response that further supports tissue regeneration. These findings not only shed light on alternative regenerative processes in plants but also open new possibilities for biotechnological applications aimed at improving plant resilience and recovery after injury. (Summary by Carlos González Sanz @carlosgonzsanz.bsky.social) Sci. Advances 10.1126/sciadv.adr1509
Have you ever placed a leaf from a Monstera plant in a glass of water and watched it grow new roots, forming a new plant? This happens because plants have a much greater ability to regenerate their tissues compared to animals. Recently, in Ruiz-Solaní et al., Spanish researchers have found a way to improve this regeneration, applying bacterial cellulose (BC) to plant wounds. BC is a biopolymer produced by specific bacterial strains that has been widely utilized in human biomedical applications as a scaffold for tissue regeneration. They found that applying bacterial cellulose to artificially cut leaves improved tissue regeneration—in other words, the cuts healed. Additionally, the compound contains plant hormones, such as cytokinin and auxins, which promote cell proliferation and differentiation. Additionally, BC activates plant defense mechanisms against microbial threats, particularly by inducing a reactive oxygen species (ROS) burst, a key response that further supports tissue regeneration. These findings not only shed light on alternative regenerative processes in plants but also open new possibilities for biotechnological applications aimed at improving plant resilience and recovery after injury. (Summary by Carlos González Sanz @carlosgonzsanz.bsky.social) Sci. Advances 10.1126/sciadv.adr1509
ATP-citrate lyase B1 regulates pollen development in Arabidopsis
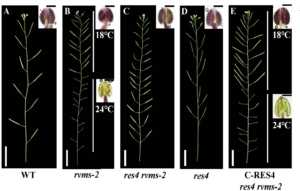 The genetic regulation of male fertility in Arabidopsis thaliana involves multiple factors, including ATP-Citrate Lyase (ACL), a key enzyme in cytoplasmic acetyl-CoA production. The thermosensitive male-sterile mutant reversible male sterile-2 (rvms-2) exhibits normal fertility at low temperatures but becomes male sterile at higher temperatures. This mutant shows defective exine formation and cytoplasmic leakage during pollen development. RVMS encodes a GDSL lipase/hydrolase protein with lipase activity, essential for proper pollen wall formation. Previous studies identified several rvms-2 suppressor mutants that restore fertility. Among these, restorer-2 (res2) and restorer-3 (res3) encode cell-wall-modifying enzymes that delay degradation of the pectin and callose walls, thereby improving pollen viability. In this new study, Ma et al. characterized restorer-4 (res4), another suppressor mutant of rvms-2, revealing that RES4 encodes ATP-Citrate Lyase B1 (ACLB1), a subunit of ACL that catalyzes the conversion of citrate to acetyl-CoA. The res4 mutation alters the citryl-CoA lyase domain of ACLB1, leading to defective exine formation and reduced microspore size. RES4 is expressed in the tapetum and regulated by the transcription factor MS188, a key player in sporopollenin synthesis. Lipid staining assays indicate significantly reduced lipid accumulation in the tapetum and locules of res4, linking ACL activity to sporopollenin production. The smaller microspore size in res4 contributes to partial restoration of fertility in rvms-2, suggesting that reduced microspore volume lowers the requirement for protective cell wall structures. This study underscores the importance of ACL-derived cytoplasmic acetyl-CoA in pollen development and offers new insights into fertility restoration mechanisms, with potential implications for hybrid crop breeding strategies. (Summary by Muhammad Aamir Khan @MAKNature1998) Plant Physiol. 10.1093/plphys/kiaf044
The genetic regulation of male fertility in Arabidopsis thaliana involves multiple factors, including ATP-Citrate Lyase (ACL), a key enzyme in cytoplasmic acetyl-CoA production. The thermosensitive male-sterile mutant reversible male sterile-2 (rvms-2) exhibits normal fertility at low temperatures but becomes male sterile at higher temperatures. This mutant shows defective exine formation and cytoplasmic leakage during pollen development. RVMS encodes a GDSL lipase/hydrolase protein with lipase activity, essential for proper pollen wall formation. Previous studies identified several rvms-2 suppressor mutants that restore fertility. Among these, restorer-2 (res2) and restorer-3 (res3) encode cell-wall-modifying enzymes that delay degradation of the pectin and callose walls, thereby improving pollen viability. In this new study, Ma et al. characterized restorer-4 (res4), another suppressor mutant of rvms-2, revealing that RES4 encodes ATP-Citrate Lyase B1 (ACLB1), a subunit of ACL that catalyzes the conversion of citrate to acetyl-CoA. The res4 mutation alters the citryl-CoA lyase domain of ACLB1, leading to defective exine formation and reduced microspore size. RES4 is expressed in the tapetum and regulated by the transcription factor MS188, a key player in sporopollenin synthesis. Lipid staining assays indicate significantly reduced lipid accumulation in the tapetum and locules of res4, linking ACL activity to sporopollenin production. The smaller microspore size in res4 contributes to partial restoration of fertility in rvms-2, suggesting that reduced microspore volume lowers the requirement for protective cell wall structures. This study underscores the importance of ACL-derived cytoplasmic acetyl-CoA in pollen development and offers new insights into fertility restoration mechanisms, with potential implications for hybrid crop breeding strategies. (Summary by Muhammad Aamir Khan @MAKNature1998) Plant Physiol. 10.1093/plphys/kiaf044
Symbiotic secrets: A multi-omics exploration of the lichen Xanthoria parietina
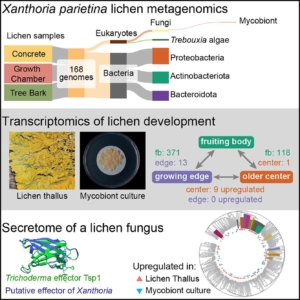 Lichens are complex symbiotic organisms formed by algae or cyanobacteria living within the filaments of multiple fungal species. This unique partnership, which is both stable and self-replicating across generations, represents one of the most successful lifestyle strategies in the biological world. Lichens play a crucial role in nutrient cycling and ecosystem stability, often thriving in extreme environments where few other organisms can survive. Interestingly, lichens exhibit properties distinct from those of their individual components, yet the molecular mechanisms underlying their formation and persistence remain largely unknown. To address this knowledge gap, Tagirdzhanova and colleagues employed metagenomics and metatranscriptomics to investigate the symbiosis in the model lichen Xanthoria parietina. Their study generated a comprehensive dataset encompassing the genome, transcriptome, and secretome, providing a valuable resource for future research on lichen developmental biology. Notably, they identified an enrichment of NLR-like genes and effector proteins in the mycobiont, suggesting that lichen symbiosis involves bidirectional recognition between symbionts and potentially the selective recruitment of compatible microbial partners. (Summary by Ching Chan @ntnuchanlab) Current Biology 10.1016/j.cub.2024.12.041
Lichens are complex symbiotic organisms formed by algae or cyanobacteria living within the filaments of multiple fungal species. This unique partnership, which is both stable and self-replicating across generations, represents one of the most successful lifestyle strategies in the biological world. Lichens play a crucial role in nutrient cycling and ecosystem stability, often thriving in extreme environments where few other organisms can survive. Interestingly, lichens exhibit properties distinct from those of their individual components, yet the molecular mechanisms underlying their formation and persistence remain largely unknown. To address this knowledge gap, Tagirdzhanova and colleagues employed metagenomics and metatranscriptomics to investigate the symbiosis in the model lichen Xanthoria parietina. Their study generated a comprehensive dataset encompassing the genome, transcriptome, and secretome, providing a valuable resource for future research on lichen developmental biology. Notably, they identified an enrichment of NLR-like genes and effector proteins in the mycobiont, suggesting that lichen symbiosis involves bidirectional recognition between symbionts and potentially the selective recruitment of compatible microbial partners. (Summary by Ching Chan @ntnuchanlab) Current Biology 10.1016/j.cub.2024.12.041
Finding balance: How plants achieve signal specificity in stomatal development and defense
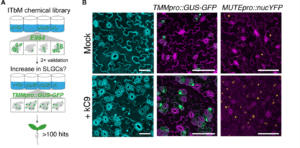 Plants need to develop and grow within their environment whilst defending themselves against potential external threats. There are limited resources for these energy-intensive processes and so cross-talk is common between the signalling pathways to find balance between growth and defence. Hermann et al., provide evidence for cross-regulation between stomatal development and the immune response and suggest that that the key to signal specificity is the balance of activation of signal receptors. Through conducting an intensive chemical screen, Hermann et al. pin-pointed a candidate signalling chemical (kC9) which activates the differentiation of stomatal cells by inhibiting the EPF-ERECTA signalling pathway. Docking models, interferometry and calorimetry assays showed that kC9 binds to the transducers of the signalling cascade, the mitogen-activated protein kinases (MAPKs), specifically MPK3 and MPK6, which inhibit stomatal development. MPK3 and MPK6 are also downstream of the FLS2-flg22 signalling pathway, a well-documented pathway in the plant immune response. flg22 treatment negated the proliferative effect of kC9, as well as the loss of ERECTA family transporters, on stomatal development, demonstrating cross-regulation from the immune pathway. This research highlights the importance of the availability of upstream receptors for signal specificity where there are overlapping components of the pathways. (Summary by Kes Maio @kesmaio.bsky.social) Science Advances 10.1126/sciadv.ads3718
Plants need to develop and grow within their environment whilst defending themselves against potential external threats. There are limited resources for these energy-intensive processes and so cross-talk is common between the signalling pathways to find balance between growth and defence. Hermann et al., provide evidence for cross-regulation between stomatal development and the immune response and suggest that that the key to signal specificity is the balance of activation of signal receptors. Through conducting an intensive chemical screen, Hermann et al. pin-pointed a candidate signalling chemical (kC9) which activates the differentiation of stomatal cells by inhibiting the EPF-ERECTA signalling pathway. Docking models, interferometry and calorimetry assays showed that kC9 binds to the transducers of the signalling cascade, the mitogen-activated protein kinases (MAPKs), specifically MPK3 and MPK6, which inhibit stomatal development. MPK3 and MPK6 are also downstream of the FLS2-flg22 signalling pathway, a well-documented pathway in the plant immune response. flg22 treatment negated the proliferative effect of kC9, as well as the loss of ERECTA family transporters, on stomatal development, demonstrating cross-regulation from the immune pathway. This research highlights the importance of the availability of upstream receptors for signal specificity where there are overlapping components of the pathways. (Summary by Kes Maio @kesmaio.bsky.social) Science Advances 10.1126/sciadv.ads3718
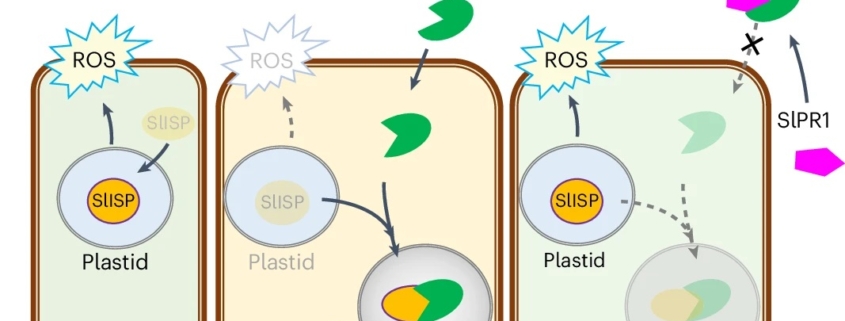
Tomato PR1 protein prevents the fungal effector FolSvp2 from suppressing SlISP-mediated ROS production
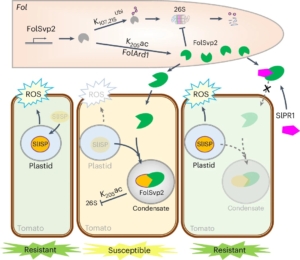 Plants and pathogens are locked in a co-evolutionary arms race. Pathogens secrete effectors into plants, leading to effector-triggered susceptibility. Plants in turn respond through the production of reactive oxygen species (ROS) and the expression of defence-related genes to defend themselves, which leads to effector-triggered immunity. Recently, Li et al. showed that FolSvp2, an effector secreted from the fungal pathogen Fusarium oxysporom f. sp. Lycopersici (Fol) undergoes modification through acetylation. This modification stabilizes this effector through phase separation inside the host cell. Furthermore, the stabilized FolSvp2 recruits into the effector condensates a tomato iron-sulfur protein, SlISP, which is normally plastid-localized and contributes to the defensive ROS burst. This interaction with FolSvp2 prevents SlISP from producing ROS, much like a thief (FolSvp2) holds a householder (SlISP) hostage and prevents it from shouting for help. The authors further showed how SlPR1, a resistance protein in tomato, prevents the condensation of SlISP by interacting with FolSvp2 in the apoplast (like the police blocking a thief from entering the household). Thus, SlPR1 prevents FolSvp2 from sequestering and inactivating SlISP, which then allows defensive ROS production. Their results show a clear example of how pathogens can use non-enzymatic approach to suppress host defenses, and that pathogen effectors also use phase separation, forming condensates within host cells during infection. (Summary by Nathaniel Oragbon @NathanIgwe) Nature Plants 10.1038/s41477-024-01811-y
Plants and pathogens are locked in a co-evolutionary arms race. Pathogens secrete effectors into plants, leading to effector-triggered susceptibility. Plants in turn respond through the production of reactive oxygen species (ROS) and the expression of defence-related genes to defend themselves, which leads to effector-triggered immunity. Recently, Li et al. showed that FolSvp2, an effector secreted from the fungal pathogen Fusarium oxysporom f. sp. Lycopersici (Fol) undergoes modification through acetylation. This modification stabilizes this effector through phase separation inside the host cell. Furthermore, the stabilized FolSvp2 recruits into the effector condensates a tomato iron-sulfur protein, SlISP, which is normally plastid-localized and contributes to the defensive ROS burst. This interaction with FolSvp2 prevents SlISP from producing ROS, much like a thief (FolSvp2) holds a householder (SlISP) hostage and prevents it from shouting for help. The authors further showed how SlPR1, a resistance protein in tomato, prevents the condensation of SlISP by interacting with FolSvp2 in the apoplast (like the police blocking a thief from entering the household). Thus, SlPR1 prevents FolSvp2 from sequestering and inactivating SlISP, which then allows defensive ROS production. Their results show a clear example of how pathogens can use non-enzymatic approach to suppress host defenses, and that pathogen effectors also use phase separation, forming condensates within host cells during infection. (Summary by Nathaniel Oragbon @NathanIgwe) Nature Plants 10.1038/s41477-024-01811-y
Shaping pathogenicity: How CRISPR-Cas loss fuels Xanthomonas evolution
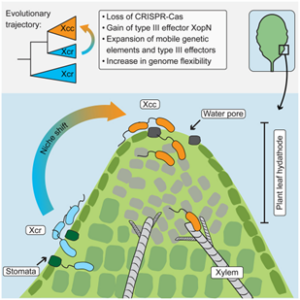 Pathogens have evolved diverse infection strategies governed by virulence factors, often targeting specific host organs or tissues. Genome fluidity plays a crucial role in enabling microbial pathogens to adapt to the dynamic selection pressures imposed by co-evolution with their hosts. In a recent study, Paauw and colleagues used a pangenome approach to investigate the genetic factors and evolutionary processes shaping the evolution of pathogenic Xanthomonas campestris (Xc) in a collection of over 180 isolates from diseased Brassica plants. Notably, different Xc pathovars exhibit distinct tissue specificities within the same leaf: Xc pv. campestris (Xcc) infects the vasculature, whereas Xc pv. raphanin (Xcr) enters leaves exclusively through stomata, like most bacteria. However, the genetic mechanisms driving the diversification of virulence factors remain unclear. Prokaryotic CRISPR-Cas systems serve as adaptive immune defenses that restrict horizontal gene transfer and limit the uptake of foreign DNA. The authors found that loss of CRISPR-Cas is more prominent in the Xcc genome compared to Xcr, facilitating the acquisition of virulence factors, and increasing genome plasticity. This enhanced genomic flexibility allows Xcc to colonize additional plant tissues that other pathovars cannot. While gene regulations can occur rapidly during host infection, the emergence of new species or subspecies requires continuous genome evolution. Overall, these findings suggest that CRISPR-Cas loss has been a key driver of Xc evolution, contributing to the emergence of agronomically important vascular pathogens in cabbage. (Summary by Ching Chan @ntnuchanlab) Curr. Biol. 10.1016/j.cub.2025.01.003
Pathogens have evolved diverse infection strategies governed by virulence factors, often targeting specific host organs or tissues. Genome fluidity plays a crucial role in enabling microbial pathogens to adapt to the dynamic selection pressures imposed by co-evolution with their hosts. In a recent study, Paauw and colleagues used a pangenome approach to investigate the genetic factors and evolutionary processes shaping the evolution of pathogenic Xanthomonas campestris (Xc) in a collection of over 180 isolates from diseased Brassica plants. Notably, different Xc pathovars exhibit distinct tissue specificities within the same leaf: Xc pv. campestris (Xcc) infects the vasculature, whereas Xc pv. raphanin (Xcr) enters leaves exclusively through stomata, like most bacteria. However, the genetic mechanisms driving the diversification of virulence factors remain unclear. Prokaryotic CRISPR-Cas systems serve as adaptive immune defenses that restrict horizontal gene transfer and limit the uptake of foreign DNA. The authors found that loss of CRISPR-Cas is more prominent in the Xcc genome compared to Xcr, facilitating the acquisition of virulence factors, and increasing genome plasticity. This enhanced genomic flexibility allows Xcc to colonize additional plant tissues that other pathovars cannot. While gene regulations can occur rapidly during host infection, the emergence of new species or subspecies requires continuous genome evolution. Overall, these findings suggest that CRISPR-Cas loss has been a key driver of Xc evolution, contributing to the emergence of agronomically important vascular pathogens in cabbage. (Summary by Ching Chan @ntnuchanlab) Curr. Biol. 10.1016/j.cub.2025.01.003
LecRK-V and trehalose-associated immunity: a conserved defense mechanism across kingdoms
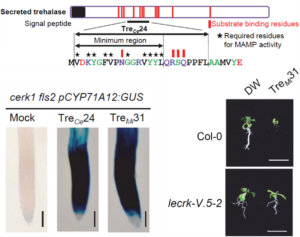 Membrane-localized receptor-like kinases (RLKs) are essential for perceiving diverse signaling molecules, including proteins, polysaccharides, hormones, reactive oxygen species, ions, and damage-associated molecular patterns. Among the well-characterized RLKs, FLS2, EFR, and CERK1 are known to recognize bacterial and fungal molecular patterns, triggering plant immune responses. However, the Arabidopsis genome encodes approximately 600 RLKs, the majority of which remain functionally uncharacterized. In a recent study, Iino, Kadota, and colleagues investigated how Arabidopsis perceives nematodes, uncovering a trehalase-derived peptide that activates immune responses in the Col-0 ecotype but not in Cvi-0. Through genetic crosses and QTL mapping, the authors identified members of the LecRK-V family as receptors for this peptide. Intriguingly, this ligand-receptor pair is evolutionarily conserved across insects and fungal pathogens, underscoring the broad-spectrum role in plant defense. This study highlights the critical function of the LecRK-V family in pathogen perception and suggests that trehalase/trehalose-associated components serve as conserved immune signals across different biological kingdoms. (Summary by Ching Chan @ntnuchanlab) bioRxiv 10.1101/2025.02.02.636166
Membrane-localized receptor-like kinases (RLKs) are essential for perceiving diverse signaling molecules, including proteins, polysaccharides, hormones, reactive oxygen species, ions, and damage-associated molecular patterns. Among the well-characterized RLKs, FLS2, EFR, and CERK1 are known to recognize bacterial and fungal molecular patterns, triggering plant immune responses. However, the Arabidopsis genome encodes approximately 600 RLKs, the majority of which remain functionally uncharacterized. In a recent study, Iino, Kadota, and colleagues investigated how Arabidopsis perceives nematodes, uncovering a trehalase-derived peptide that activates immune responses in the Col-0 ecotype but not in Cvi-0. Through genetic crosses and QTL mapping, the authors identified members of the LecRK-V family as receptors for this peptide. Intriguingly, this ligand-receptor pair is evolutionarily conserved across insects and fungal pathogens, underscoring the broad-spectrum role in plant defense. This study highlights the critical function of the LecRK-V family in pathogen perception and suggests that trehalase/trehalose-associated components serve as conserved immune signals across different biological kingdoms. (Summary by Ching Chan @ntnuchanlab) bioRxiv 10.1101/2025.02.02.636166