Plant Science Research Weekly: December 20, 2024
When form fits function: the value of root diversity to survival
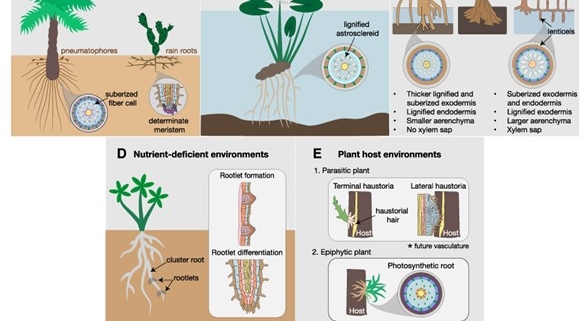
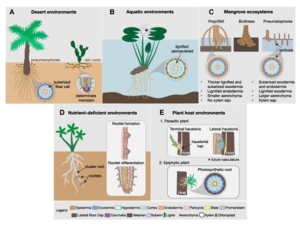 For a plant, form is function. Despite the diversity of forms that exist in nature, plant root diversity is notoriously understudied compared to their aerial counterpart. This review by Ramachandran and Ramirez et al. aims to revitalize the field of root form-function research by accentuating the vast array of root adaptations plants have evolved to survive different ecological niches. The review covers root specialization at different organizational levels from whole root systems to cellular modifications. From drought to hypoxia, plants have to endure a multitude of environmental stresses in nature and have developed highly specialized strategies to mitigate abiotic and biotic challenges. The authors underline the importance of understanding at a mechanistic level how root patterning and cell type composition can influence plant growth and resilience to environmental factors. Naturally, altering root development requires an intimate knowledge of the molecular players governing the root developmental program, including genetic and hormonal pathways. There is value in harnessing knowledge from biodiversity, in already existing root adaptations, and root form-function research can provide potentially transferable knowledge to benefit crop cultivation and plant conservation. (Summary by Marvin Jin @MarvinJYS) Plant Physiol. 10.1093/plphys/kiae586
For a plant, form is function. Despite the diversity of forms that exist in nature, plant root diversity is notoriously understudied compared to their aerial counterpart. This review by Ramachandran and Ramirez et al. aims to revitalize the field of root form-function research by accentuating the vast array of root adaptations plants have evolved to survive different ecological niches. The review covers root specialization at different organizational levels from whole root systems to cellular modifications. From drought to hypoxia, plants have to endure a multitude of environmental stresses in nature and have developed highly specialized strategies to mitigate abiotic and biotic challenges. The authors underline the importance of understanding at a mechanistic level how root patterning and cell type composition can influence plant growth and resilience to environmental factors. Naturally, altering root development requires an intimate knowledge of the molecular players governing the root developmental program, including genetic and hormonal pathways. There is value in harnessing knowledge from biodiversity, in already existing root adaptations, and root form-function research can provide potentially transferable knowledge to benefit crop cultivation and plant conservation. (Summary by Marvin Jin @MarvinJYS) Plant Physiol. 10.1093/plphys/kiae586
Opinion: Resilient plants for a sustainable future
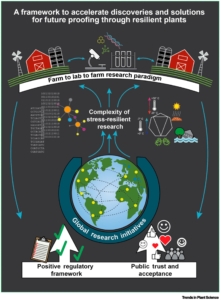 Anthropogenic climate change has introduced tremendous uncertainty about the future of all life on Earth. As primary producers, threats to plants are transduced up the food chain to their consumers, including people, which is why there is a great need to develop resilient plants that are able to sustain us in spite of these new challenges. A new article by Rhee et al. lays out a set of recommendations, addressing both plant breeding and plant cropping systems, to accelerate the development of climate resilient crops. First, they call for greater international cooperation particularly between the Global North and Global South, but also increased public-private cooperation. Second, they argue that research must leverage the power of reductionist tools such as biochemistry and genetics to address plants as complex systems that live in complex environments. They advocate for a connected global network of outdoor phenotyping facilities where genotypes can be tested in diverse climates. Third, the authors call for greater communication and cooperation between those who study plants in the lab, the field, and the farm. Their fourth recommendation is to address public concerns about new technologies through better communication and public engagement. And finally, they observe that regulation of new crops must be science-based, rapid, and streamlined. (Summary by Mary Williams @PlantTeaching.bsky.social @PlantTeaching) Trends Plant Sci. 10.1016/j.tplants.2024.11.001
Anthropogenic climate change has introduced tremendous uncertainty about the future of all life on Earth. As primary producers, threats to plants are transduced up the food chain to their consumers, including people, which is why there is a great need to develop resilient plants that are able to sustain us in spite of these new challenges. A new article by Rhee et al. lays out a set of recommendations, addressing both plant breeding and plant cropping systems, to accelerate the development of climate resilient crops. First, they call for greater international cooperation particularly between the Global North and Global South, but also increased public-private cooperation. Second, they argue that research must leverage the power of reductionist tools such as biochemistry and genetics to address plants as complex systems that live in complex environments. They advocate for a connected global network of outdoor phenotyping facilities where genotypes can be tested in diverse climates. Third, the authors call for greater communication and cooperation between those who study plants in the lab, the field, and the farm. Their fourth recommendation is to address public concerns about new technologies through better communication and public engagement. And finally, they observe that regulation of new crops must be science-based, rapid, and streamlined. (Summary by Mary Williams @PlantTeaching.bsky.social @PlantTeaching) Trends Plant Sci. 10.1016/j.tplants.2024.11.001
Formation of plasmodesmata bridges through ER-dependent incomplete cytokinesis
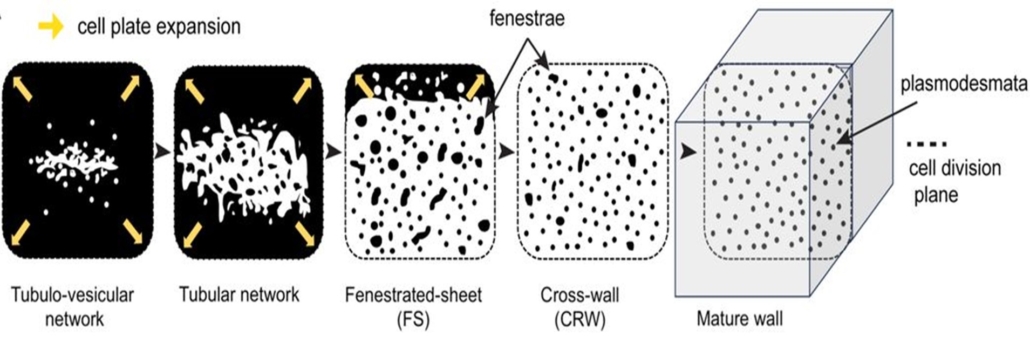 Plasmodesmata are important for intercellular communication in plants. They are formed through incomplete cytokinesis during which there is no “final cut” of the communication between daughter cells. Unlike animal cells that have a single bridge between cells, plants create several hundreds of plasmodesmata bridges between daughter cells. While the function and structure of the plasmodesmata has been known for many years, the formation process of the plasmodesmata has been a mystery and this is what Li et al. seek to uncover. Using the model plant Arabidopsis thaliana, the authors used high-resolution imaging techniques to map the transition from cell plate formation to the formation of the plasmodesmata. They showed that the endoplasmic reticulum (ER) serves as the “architect”, as it moves through fenestrae (holes) in the cell plate. As the cell plate matures to become the plasmodesmata, fenestrae (holes) without the ER are shut off, while those with ER cell-cell continuity eventually become the nascent plasmodesmata, leading to bridge formation, hence, communication between cells. As with any literal bridge construction, the ER-PM protein tethers MCTP3, MCTP4 and MCTP6 act as the “construction workers” needed to stabilize this bridge between cells. (Summary by Nathaniel Oragbon @NathanIgwe) Science 10.1126/science.adn4630
Plasmodesmata are important for intercellular communication in plants. They are formed through incomplete cytokinesis during which there is no “final cut” of the communication between daughter cells. Unlike animal cells that have a single bridge between cells, plants create several hundreds of plasmodesmata bridges between daughter cells. While the function and structure of the plasmodesmata has been known for many years, the formation process of the plasmodesmata has been a mystery and this is what Li et al. seek to uncover. Using the model plant Arabidopsis thaliana, the authors used high-resolution imaging techniques to map the transition from cell plate formation to the formation of the plasmodesmata. They showed that the endoplasmic reticulum (ER) serves as the “architect”, as it moves through fenestrae (holes) in the cell plate. As the cell plate matures to become the plasmodesmata, fenestrae (holes) without the ER are shut off, while those with ER cell-cell continuity eventually become the nascent plasmodesmata, leading to bridge formation, hence, communication between cells. As with any literal bridge construction, the ER-PM protein tethers MCTP3, MCTP4 and MCTP6 act as the “construction workers” needed to stabilize this bridge between cells. (Summary by Nathaniel Oragbon @NathanIgwe) Science 10.1126/science.adn4630
Plant eyes in the dark: How a blue-light photoreceptor senses and functions without light
 Light is both a source of energy for photosynthesis and a key environmental signal that regulates plant growth. Seedlings grown in darkness exhibit elongated hypocotyls and shorter roots, while light promotes shorter hypocotyls and longer roots. Cryptochromes (CRYs), as blue-light receptors, mediate many blue-light-dependent processes, but their roles under darkness or non-blue-light conditions remain unclear. Zeng et al. discovered that Arabidopsis CRY2 suppresses root elongation in darkness by inhibiting cell division in the root apical meristem. Under blue light, CRY2 forms oligomers, which disrupt this suppression, thereby promoting root cell division and elongation. This study reveals that CRY2 retains biological activity in its non-light-activated state, with blue light effectively “switching off” this activity to regulate root development. In darkness, CRY2 interacts and inhibits the activities of FORKED-LIKE (FL) proteins that promote cell division by enhancing the transcription of division-related genes. In contrast, blue-light-induced CRY2 oligomerization prevents this interaction, releasing FL activity to stimulate root growth. By integrating light and dark cues through this dual-regulation mechanism, plants achieve a balance between aboveground and underground development. This study answers the long-standing question of CRY2’s function in darkness, revealing its “darkness-dependent” activity alongside its known “blue-light-dependent” role. It expands our understanding of photoreceptor biology and offers new strategies to regulate crop growth and architecture through photoreceptor manipulation. (Summary by Hao Chen) Science doi.org/10.1016/j.cell.2024.10.031
Light is both a source of energy for photosynthesis and a key environmental signal that regulates plant growth. Seedlings grown in darkness exhibit elongated hypocotyls and shorter roots, while light promotes shorter hypocotyls and longer roots. Cryptochromes (CRYs), as blue-light receptors, mediate many blue-light-dependent processes, but their roles under darkness or non-blue-light conditions remain unclear. Zeng et al. discovered that Arabidopsis CRY2 suppresses root elongation in darkness by inhibiting cell division in the root apical meristem. Under blue light, CRY2 forms oligomers, which disrupt this suppression, thereby promoting root cell division and elongation. This study reveals that CRY2 retains biological activity in its non-light-activated state, with blue light effectively “switching off” this activity to regulate root development. In darkness, CRY2 interacts and inhibits the activities of FORKED-LIKE (FL) proteins that promote cell division by enhancing the transcription of division-related genes. In contrast, blue-light-induced CRY2 oligomerization prevents this interaction, releasing FL activity to stimulate root growth. By integrating light and dark cues through this dual-regulation mechanism, plants achieve a balance between aboveground and underground development. This study answers the long-standing question of CRY2’s function in darkness, revealing its “darkness-dependent” activity alongside its known “blue-light-dependent” role. It expands our understanding of photoreceptor biology and offers new strategies to regulate crop growth and architecture through photoreceptor manipulation. (Summary by Hao Chen) Science doi.org/10.1016/j.cell.2024.10.031
Cambium secrets for vascular stem cell precision and adaptability
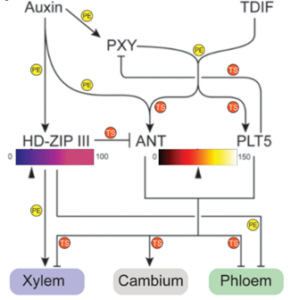 The vascular cambium, a bifacial stem cell niche, generates xylem on one side and phloem on the other, driving wood formation—the largest reservoir of terrestrial biomass. This developmental system must balance producing two distinct cell types while maintaining a reservoir of stem cells. Key questions remain: How are cambial stem cells positioned and maintained, and how does the cambium dynamically regulate its size and organization to support growth while preserving functional integrity? Auxin signaling, along with the ligand TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR (TDIF) and its receptor PHLOEM INTERCALATED WITH XYLEM (PXY), has been implicated in stem cell maintenance. However, the mechanisms connecting these components to precise stem cell regulation were unclear. Eswaran et al. identified CAMBIUM-EXPRESSED AINTEGUMENTA-LIKE (CAIL) transcription factors as key players in cambium stem cell identity through transcriptomic analysis of TDIF-overexpressing plants and PXY mutants. Their findings reveal that CAIL expression is restricted to cambial stem cells through a sequestration-based mechanism, where strong binding of TDIF to PXY halts TDIF diffusion at the edge of the auxin-PXY gradient. This spatial restriction creates a narrow signaling domain, ensuring robust CAIL expression and precise stem cell positioning. Disruption of this system—via TDIF overproduction or PXY knockdown—leads to an expansion of CAIL expression into the xylem domain, disrupting stem cell niche organization. This sequestration mechanism also allows dynamic adjustment of cambium size during growth. By employing opposing morphogen gradients and feedback mechanisms, plants, like animals, achieve precise control over stem cell positioning and fate decisions. This study offers a molecular framework for understanding how the cambium balances stability and adaptability in vascular development, advancing insights into stem cell niche regulation. (Summary by Hao Chen) Science 10.1126/science.adj8752
The vascular cambium, a bifacial stem cell niche, generates xylem on one side and phloem on the other, driving wood formation—the largest reservoir of terrestrial biomass. This developmental system must balance producing two distinct cell types while maintaining a reservoir of stem cells. Key questions remain: How are cambial stem cells positioned and maintained, and how does the cambium dynamically regulate its size and organization to support growth while preserving functional integrity? Auxin signaling, along with the ligand TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR (TDIF) and its receptor PHLOEM INTERCALATED WITH XYLEM (PXY), has been implicated in stem cell maintenance. However, the mechanisms connecting these components to precise stem cell regulation were unclear. Eswaran et al. identified CAMBIUM-EXPRESSED AINTEGUMENTA-LIKE (CAIL) transcription factors as key players in cambium stem cell identity through transcriptomic analysis of TDIF-overexpressing plants and PXY mutants. Their findings reveal that CAIL expression is restricted to cambial stem cells through a sequestration-based mechanism, where strong binding of TDIF to PXY halts TDIF diffusion at the edge of the auxin-PXY gradient. This spatial restriction creates a narrow signaling domain, ensuring robust CAIL expression and precise stem cell positioning. Disruption of this system—via TDIF overproduction or PXY knockdown—leads to an expansion of CAIL expression into the xylem domain, disrupting stem cell niche organization. This sequestration mechanism also allows dynamic adjustment of cambium size during growth. By employing opposing morphogen gradients and feedback mechanisms, plants, like animals, achieve precise control over stem cell positioning and fate decisions. This study offers a molecular framework for understanding how the cambium balances stability and adaptability in vascular development, advancing insights into stem cell niche regulation. (Summary by Hao Chen) Science 10.1126/science.adj8752
Regulation and function of a polarly localized lignin barrier in the exodermis
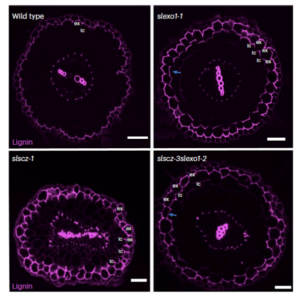 The endodermal Casparian strip (CS) is a conserved lignin-based barrier in plant roots that seals the endodermal apoplastic space. Most angiosperms possess an additional root cell type, the exodermis, which also forms a barrier. However, its regulation remains poorly understood as this cell type is absent in Arabidopsis thaliana. In tomato (Solanum lycopersicum), Manzano et al. identified a distinct exodermal structure, the polar lignin cap (PLC), which mirrors the function of the CS but is regulated by distinct genetic mechanisms. Through histochemical staining and transmission electron microscopy, the authors confirmed the PLC’s role as an apoplastic barrier. They further demonstrated its functional equivalence to the CS using propidium iodide as a tracer to assess barrier integrity. However, tomato mutant analyses of orthologues of Arabidopsis CS regulators revealed that genetic pathways governing exodermal and endodermal barriers are distinct. By mining gene expression data and analyzing mutants, the researchers identified transcription factors SlSCZ and SlEXO1 as key repressors of PLC formation in inner cortical layers, orchestrating its polar deposition and regulating downstream genes. Despite shared roles as mineral ion checkpoints, the PLC cannot fully substitute the CS’s function in ion regulation, underscoring the specialized and non-redundant roles of these barriers in maintaining root ion homeostasis. This study provides insights into the genetic regulation of the exodermis but also highlights its critical role in water and nutrient transport, broadening our understanding of plant root-environment interactions. (Summary by Elisa De Meo) Nature Plants 10.1038/s41477-024-01864-z
The endodermal Casparian strip (CS) is a conserved lignin-based barrier in plant roots that seals the endodermal apoplastic space. Most angiosperms possess an additional root cell type, the exodermis, which also forms a barrier. However, its regulation remains poorly understood as this cell type is absent in Arabidopsis thaliana. In tomato (Solanum lycopersicum), Manzano et al. identified a distinct exodermal structure, the polar lignin cap (PLC), which mirrors the function of the CS but is regulated by distinct genetic mechanisms. Through histochemical staining and transmission electron microscopy, the authors confirmed the PLC’s role as an apoplastic barrier. They further demonstrated its functional equivalence to the CS using propidium iodide as a tracer to assess barrier integrity. However, tomato mutant analyses of orthologues of Arabidopsis CS regulators revealed that genetic pathways governing exodermal and endodermal barriers are distinct. By mining gene expression data and analyzing mutants, the researchers identified transcription factors SlSCZ and SlEXO1 as key repressors of PLC formation in inner cortical layers, orchestrating its polar deposition and regulating downstream genes. Despite shared roles as mineral ion checkpoints, the PLC cannot fully substitute the CS’s function in ion regulation, underscoring the specialized and non-redundant roles of these barriers in maintaining root ion homeostasis. This study provides insights into the genetic regulation of the exodermis but also highlights its critical role in water and nutrient transport, broadening our understanding of plant root-environment interactions. (Summary by Elisa De Meo) Nature Plants 10.1038/s41477-024-01864-z
Capping your occupancy: programmed cell death as a mechanism to restrict microbial colonization of the root tip
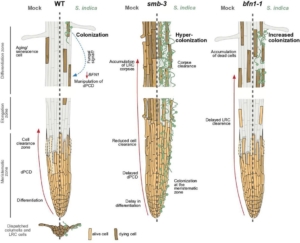 Thanks to the continued shedding and renewal of root cap cells, plant roots are able to extend into further reaches within the soil column overcoming physical barriers and potential microbial attacks, or so we assumed. Charura et al. explored the latter hypothesis showing that timely programmed cell death at the root cap confers protective properties to the model plant Arabidopsis. They found that two proteins, SMB and BFN1, are involved in regulating cell death and post-mortem cell removal, and in turn regulate plant-microbe interactions. Loss-of function smb-3 and bfn1-1 mutant roots both revealed an accumulation of cell corpses with aggregated proteins along the root. The authors then tested root colonization of the root endophytic fungus, Serendipita indica, in both mutant plants. The increased, uncleared cell corpses in smb-3 mutant roots induced fungal hypercolonization, while bfn1-1 mutants displayed increased S. indica biomass in later stages of interaction. From these findings, the authors proposed that SMB-mediated cell corpse clearance and BFN1 downregulation facilitates root fungal accommodation. Notably, the authors also inoculated Arabidopsis roots with other beneficial microbes. These interactions also yielded the consistent downregulation of BFN1 showing that this mechanism is a conserved strategy employed by microbes to exert transcriptional control to alter cell death program in plants to improve colonization. (Summary by Marvin Jin @MarvinJYS) eLife 10.7554/eLife.96266.3
Thanks to the continued shedding and renewal of root cap cells, plant roots are able to extend into further reaches within the soil column overcoming physical barriers and potential microbial attacks, or so we assumed. Charura et al. explored the latter hypothesis showing that timely programmed cell death at the root cap confers protective properties to the model plant Arabidopsis. They found that two proteins, SMB and BFN1, are involved in regulating cell death and post-mortem cell removal, and in turn regulate plant-microbe interactions. Loss-of function smb-3 and bfn1-1 mutant roots both revealed an accumulation of cell corpses with aggregated proteins along the root. The authors then tested root colonization of the root endophytic fungus, Serendipita indica, in both mutant plants. The increased, uncleared cell corpses in smb-3 mutant roots induced fungal hypercolonization, while bfn1-1 mutants displayed increased S. indica biomass in later stages of interaction. From these findings, the authors proposed that SMB-mediated cell corpse clearance and BFN1 downregulation facilitates root fungal accommodation. Notably, the authors also inoculated Arabidopsis roots with other beneficial microbes. These interactions also yielded the consistent downregulation of BFN1 showing that this mechanism is a conserved strategy employed by microbes to exert transcriptional control to alter cell death program in plants to improve colonization. (Summary by Marvin Jin @MarvinJYS) eLife 10.7554/eLife.96266.3
Smelly plant: What are they feeding you?
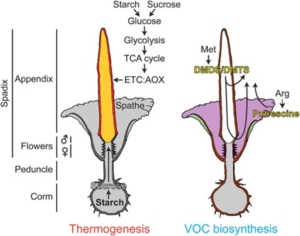 While flowers are typically associated with pleasant fragrances, every few years a certain part of the rainforests of Sumatra is filled with the pungent odor of rotting flesh. This smell emanates from the inflorescence of the titan arum, or corpse plant, which heats up during flowering in a process known as thermogenesis. Thermogenesis is rare in plants, but is thought to help produce the unpleasant scent that attracts the plant’s necro- or saprophagous pollinators (those that eat dead or decaying tissues). Zulfiqar and colleagues made use of different blooming events of a single titan arum specimen and its clonal plantlet to gain more insights into this rare phenomenon. RNA sequencing of different tissues of the spadix at peak thermogenesis was performed to understand the molecular basis of thermogenesis and volatile organic compound (VOC) production. Expression of genes related to sugar transport and starch breakdown showed significant but tissue-specific changes. This supports the idea that thermogenesis is linked to the mobilization of starch reserves from the tuber towards the appendix, which contains the flowers, where it is broken down. There it can then serve as the respiratory substrate for alternative oxidases, which are essential for thermogenesis. Additionally, genes involved in sulfur metabolism were elevated in the appendix as well, confirming their role in VOC production. Interestingly, the authors also detected, for the first time, elevated levels of putrescine in the spathe margin, which surrounds the appendix and has a supporting function. In conclusion, the authors provide new insights into the genetic and metabolic basis of thermogenesis in this unique plant. (Summary by Thomas Depaepe, Bluesky: @thdpaepe.bsky.social and X: @thdpaepe) PNAS Nexus 10.1093/pnasnexus/pgae492
While flowers are typically associated with pleasant fragrances, every few years a certain part of the rainforests of Sumatra is filled with the pungent odor of rotting flesh. This smell emanates from the inflorescence of the titan arum, or corpse plant, which heats up during flowering in a process known as thermogenesis. Thermogenesis is rare in plants, but is thought to help produce the unpleasant scent that attracts the plant’s necro- or saprophagous pollinators (those that eat dead or decaying tissues). Zulfiqar and colleagues made use of different blooming events of a single titan arum specimen and its clonal plantlet to gain more insights into this rare phenomenon. RNA sequencing of different tissues of the spadix at peak thermogenesis was performed to understand the molecular basis of thermogenesis and volatile organic compound (VOC) production. Expression of genes related to sugar transport and starch breakdown showed significant but tissue-specific changes. This supports the idea that thermogenesis is linked to the mobilization of starch reserves from the tuber towards the appendix, which contains the flowers, where it is broken down. There it can then serve as the respiratory substrate for alternative oxidases, which are essential for thermogenesis. Additionally, genes involved in sulfur metabolism were elevated in the appendix as well, confirming their role in VOC production. Interestingly, the authors also detected, for the first time, elevated levels of putrescine in the spathe margin, which surrounds the appendix and has a supporting function. In conclusion, the authors provide new insights into the genetic and metabolic basis of thermogenesis in this unique plant. (Summary by Thomas Depaepe, Bluesky: @thdpaepe.bsky.social and X: @thdpaepe) PNAS Nexus 10.1093/pnasnexus/pgae492
This is the final edition of Plant Science Resaerch Weekly in 2024! We thank all of the contributors, including many 2024 Plantae Fellows, who have summarized exciting new research from across the discipline for you this year. We wish everyone a Happy Solstice, Happy New Year, and we look forward to helping you stay on top of what’s hot in plant science in 2025!