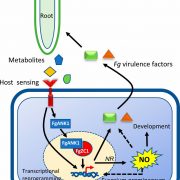
Phosphorylation-dependent sub-functionalization of the calcium-dependent protein kinase CPK28 (bioRxiv)
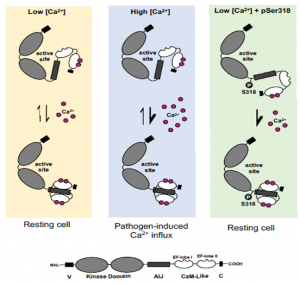 Calcium-dependent protein kinases (CPKs) form an important class of sensor-responder proteins that bind Ca2+ to ‘sense’ intracellular Ca2+ levels, and ‘respond’ by phosphorylating target proteins. CPK28 has distinct roles in growth and defense. To analyze the importance of Ca2+ in fine-tuning CPK28 function, Bredow and colleagues identified a single phosphorylation site, serine (Ser) 318, as vital for separating its dual functions in regulating immune responses and stem elongation. The authors found that mutating Ser318 into a non-phosphorylatable form leaves it unable to complement the immune response phenotype in a cpk28 mutant, indicating that Ser318 phosphorylation is necessary for immune response. In agreement, they observed that another kinase, BIK1, can phosphorylate CPK28 on Ser318 to regulate immune responses. Importantly, the authors found this site critical for phosphorylation at physiological concentrations of Ca2+, which alters the conformation of CPK28. Together with other results, they conclude that at physiological Ca2+ levels, phosphorylation of Ser318, which is conserved among a group of CPKs, is essential for “priming” the protein to sense further increase in cellular Ca2+ following a pathogen attack. (Summary by Pavithran Narayanan @pavi_narayanan) bioRxiv 10.1101/2020.10.16.338442v1
Calcium-dependent protein kinases (CPKs) form an important class of sensor-responder proteins that bind Ca2+ to ‘sense’ intracellular Ca2+ levels, and ‘respond’ by phosphorylating target proteins. CPK28 has distinct roles in growth and defense. To analyze the importance of Ca2+ in fine-tuning CPK28 function, Bredow and colleagues identified a single phosphorylation site, serine (Ser) 318, as vital for separating its dual functions in regulating immune responses and stem elongation. The authors found that mutating Ser318 into a non-phosphorylatable form leaves it unable to complement the immune response phenotype in a cpk28 mutant, indicating that Ser318 phosphorylation is necessary for immune response. In agreement, they observed that another kinase, BIK1, can phosphorylate CPK28 on Ser318 to regulate immune responses. Importantly, the authors found this site critical for phosphorylation at physiological concentrations of Ca2+, which alters the conformation of CPK28. Together with other results, they conclude that at physiological Ca2+ levels, phosphorylation of Ser318, which is conserved among a group of CPKs, is essential for “priming” the protein to sense further increase in cellular Ca2+ following a pathogen attack. (Summary by Pavithran Narayanan @pavi_narayanan) bioRxiv 10.1101/2020.10.16.338442v1