Pathogen protein modularity enables elaborate mimicry of a host phosphatase
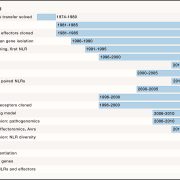
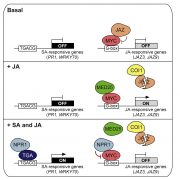
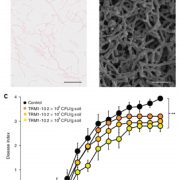
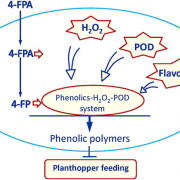
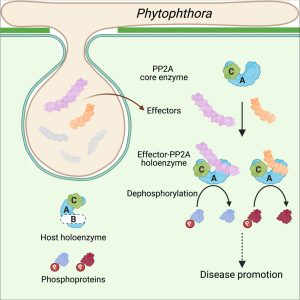 Some of the most fascinating discoveries in biology are found at the interface between hosts and pathogens, where each organism is endlessly fighting for its survival. Plant pathogens produce a wide array of effector proteins that promote their virulence, but we only understand how some of these effectors function. Here, Li et al. have uncovered how a family of effectors from the oomycete pathogen Phytophthora sojae promote disease in their hosts. Previous studies identified a large family of effector proteins with stretches of repeating units consisting of several L (Leu) residues followed by one W (Trp) and one Y (Tyr), known as (L)WY motifs. The authors found that some of these (L)WY-motif containing effectors interact with the core complex of the serine/threonine protein phosphatase 2A (PP2A) enzyme. Specifically, these effectors compete with the regulatory B domain, which specifies the dephosphorylation targets, for binding to the core complex. The presence of the effectors changes the specificity of the core phosphatase, leading to different sets of dephosphorylated proteins; different from in the absence of the effector, and different between different (L)WY-containing effectors. The authors point out that the modularity of these effector domains readily permits domain shuffling to generate new effectors conferring different specificity to the hybrid phosphastase complex, enhancing the evolvability of the effector repertoire. (Summary by Mary Williams @PlantTeaching) Cell 10.1016/j.cell.2023.05.049
Some of the most fascinating discoveries in biology are found at the interface between hosts and pathogens, where each organism is endlessly fighting for its survival. Plant pathogens produce a wide array of effector proteins that promote their virulence, but we only understand how some of these effectors function. Here, Li et al. have uncovered how a family of effectors from the oomycete pathogen Phytophthora sojae promote disease in their hosts. Previous studies identified a large family of effector proteins with stretches of repeating units consisting of several L (Leu) residues followed by one W (Trp) and one Y (Tyr), known as (L)WY motifs. The authors found that some of these (L)WY-motif containing effectors interact with the core complex of the serine/threonine protein phosphatase 2A (PP2A) enzyme. Specifically, these effectors compete with the regulatory B domain, which specifies the dephosphorylation targets, for binding to the core complex. The presence of the effectors changes the specificity of the core phosphatase, leading to different sets of dephosphorylated proteins; different from in the absence of the effector, and different between different (L)WY-containing effectors. The authors point out that the modularity of these effector domains readily permits domain shuffling to generate new effectors conferring different specificity to the hybrid phosphastase complex, enhancing the evolvability of the effector repertoire. (Summary by Mary Williams @PlantTeaching) Cell 10.1016/j.cell.2023.05.049