Location, location, location: phototropin 2 action at the chloroplast membrane
As someone with a background in plant molecular genetics, I confess to occasionally forgetting about location. Reverse genetics is a powerful tool for picking apart gene networks, but in which organ do these genes act? Which tissue of that organ? Which cells of that tissue and which compartment of those cells? These are important questions that must be addressed.
In the field of photobiology there is a growing interest in the spatial aspects of photoperception, particularly regarding the subcellular location of photoreceptors (Ronald and Davis, 2019). Of the five classes of photoreceptors, four (phytochromes, cryptochromes, UVR8, and zeitlupes) are found in the nucleus or cytoplasm. The fifth class, phototropins, are unique in that they are membrane bound. It may be this property that makes phototropins particularly adept at deciphering directional light cues.
In arabidopsis there are two phototropins (phot1 and phot2). phot1 is localised primarily at the plasma membrane but undergoes internalisation when activated by blue light. The plasma membrane appears to be the major site of phot1 action, and the role (if any) of internalised phot1 is debated (Preuten et al., 2015). phot2 is primarily bound to the plasma membrane, but it also accumulates to an appreciable level on the chloroplast outer membrane (Kong and Wada, 2016). The two phototropins redundantly regulate phototropism, leaf flattening, stomatal opening, and chloroplast movement towards low intensity light (chloroplast accumulation). The movement of chloroplasts away from high intensity light (chloroplast avoidance)) is mediated exclusively by phot2. Is the specificity of phot2 in chloroplast avoidance due then to its accumulation on chloroplast membranes?
In this issue of Plant Physiology, Ishishita et al. (2020) set out to tackle this question. They began by complementing a phot1phot2 mutant line with either wild-type phot2, or phot2 targeted to the plasma or chloroplast membranes. After confirming that these proteins were indeed restricted to the correct subcellular location, the authors analysed which phototropin-dependent responses were rescued in these lines.
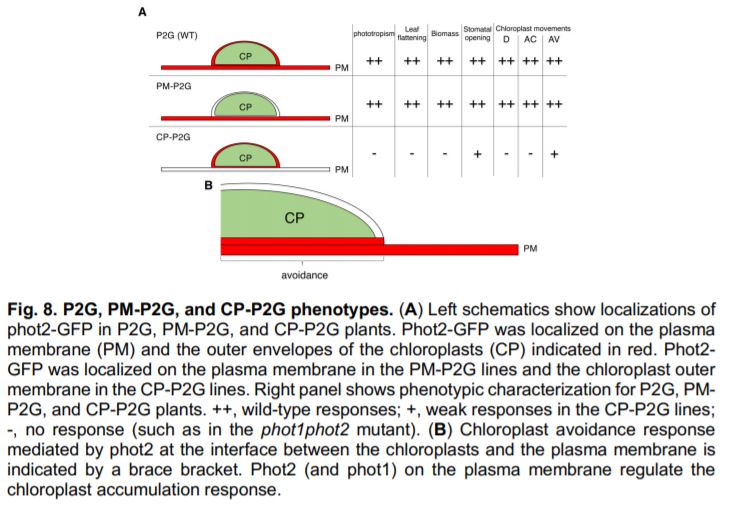 Plasma membrane-targeted phot2 completely rescued the curled leaf, non-phototropic and stomatal-opening defects of the phot1phot2 mutant, indicating that plasma membrane-localised phot2 is sufficient for all of these responses (Fig. 1A). By contrast, chloroplast membrane-targeted phot2 failed to rescue leaf flattening or phototropism. Chloroplastic phot2 did however rescue blue light-dependent stomatal opening to some extent. Why does phot2 on the chloroplast membrane rescue stomatal opening, but not leaf flattening or phototropism? One of the key phot2 binding partners required for leaf flattening and phototropism is present only on the plasma membrane (Christie et al., 2018). By contrast, blue light-mediated stomatal opening requires the interaction of phototropins with a cytosolic protein (Takemiya et al., 2013). The authors suggest that this freely moving cytosolic protein could be partially activated by chloroplast-bound phot2.
Plasma membrane-targeted phot2 completely rescued the curled leaf, non-phototropic and stomatal-opening defects of the phot1phot2 mutant, indicating that plasma membrane-localised phot2 is sufficient for all of these responses (Fig. 1A). By contrast, chloroplast membrane-targeted phot2 failed to rescue leaf flattening or phototropism. Chloroplastic phot2 did however rescue blue light-dependent stomatal opening to some extent. Why does phot2 on the chloroplast membrane rescue stomatal opening, but not leaf flattening or phototropism? One of the key phot2 binding partners required for leaf flattening and phototropism is present only on the plasma membrane (Christie et al., 2018). By contrast, blue light-mediated stomatal opening requires the interaction of phototropins with a cytosolic protein (Takemiya et al., 2013). The authors suggest that this freely moving cytosolic protein could be partially activated by chloroplast-bound phot2.
The authors also investigated how chloroplast movement is affected by phot2 localisation. They observed that chloroplast-accumulation was rescued by plasma membrane-localised phot2, but not by chloroplast-bound phot2. This finding is consistent with previous work that showed that chloroplast accumulation requires illumination of the plasma membrane, rather than illumination of the chloroplasts themselves (Kong and Wada, 2016). Chloroplast avoidance, by contrast, requires direct illumination of the chloroplast. It was therefore surprising that the group observed that plasma membrane-localised phot2 fully rescued the chloroplast-avoidance response. Chloroplastic phot2 also weakly rescued chloroplast avoidance. The authors conclude that chloroplast avoidance is driven largely by plasma membrane-bound phot2, likely at the boundary between the plasma and chloroplast membranes (Fig. 1B). This result also shows that the phot2-specific role in regulating chloroplast avoidance is not due to its chloroplastic localisation. The authors propose that other differences between phot1 and phot2 (such as substrate specificity) may underlie phot2-specific functions. It is interesting to note that the two phenotypes rescued by chloroplast-bound phot2 (stomatal opening and chloroplast-avoidance) are also the two phototropin-mediated responses that are independent of the signalling partner NPH3 (Christie et al., 2018). Assessing the significance of this observation would however require further research.
The finding that chloroplast-localised phot2 is able to somewhat induce both chloroplast avoidance and stomatal opening is interesting, but we still don’t know if it has any physiological relevance. The authors observed that plants expressing wild -type phot2 (on the plasma and chloroplast membranes) accumulated similar biomass to plants expressing only plasma membrane-localised phot2. This seems to argue that chloroplastic phot2 does not play a significant role in biomass accumulation. It should however be noted that in this experiment, plants were grown under constant light (an environment in which stomatal opening and chloroplast avoidance are not likely to play a major role). Assessing plant biomass accumulation under fluctuating light at higher intensities may yet reveal a physiologically important role for chloroplast-localised phot2.
REFERENCES
Christie JM, Suetsugu N, Sullivan S, Wada M (2018) Shining light on the function of NPH3/RPT2-like proteins in phototropin signaling. Plant Physiol 176: 1015–1024
Kong SG, Wada M (2016) Molecular basis of chloroplast photorelocation movement. J Plant Res 129: 159–166
Preuten T, Blackwood L, Christie JM, Fankhauser C (2015) Lipid anchoring of arabidopsis phototropin 1 to assess the functional significance of receptor internalization: Should I stay or should I go? New Phytol 206: 1038–1050
Ronald J, Davis SJ (2019) Focusing on the nuclear and subnuclear dynamics of light and circadian signalling. Plant Cell Environ 42: 2871–2884
Takemiya A, Sugiyama N, Fujimoto H, Tsutsumi T, Yamauchi S, Hiyama A, Tada Y, Christie JM, Shimazaki KI (2013) Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat Commun 4: 1–8