Interplay between phosphorylation and ubiquitination is key for regulating reactive oxygen species during plant immunity (Nature Comms)
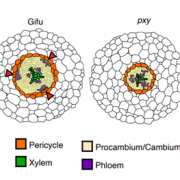
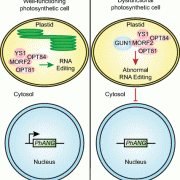
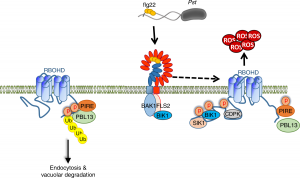 Reactive oxygen species (ROS) are essential secondary messengers for rapid transmission of local and long-distance signalling in plants. Respiratory oxidase homologs (RBOHs) are the family of enzymes in plants that produce extracellular ROS, with RBOHD being the primary oxidase in Arabidopsis for ROS production following pathogen perception. Positive regulation of RBOHD by activating phosphorylation is well characterized, but it was unknown how RHOBD is inhibited pre- and post-pathogen recognition to maintain immune homeostasis. Lee et al. identified that PBS1-like kinase 13 (PBL13) phosphorylates conserved C-terminal residues on RBOHD to decrease its stability. They further identified the previously uncharacterized ubiquitin E3 ligase PIRE (PBL13 interacting RING domain E3 ligase), which polyubiquitinates RBOHD at residues near the PBL13-targetted phosphorylation sites, targeting it for endocytosis and vacuolar degradation. Mimicking phosphorylation at these sites directly promotes ubiquitination and protein turnover. These results showcase cross-talk between phosphorylation and ubiquitination as a mechanism to negatively regulate plant immunity. (Summary by Katy Dunning @plantmomkaty) Nature Comms 10.1038/s41467-020-15601-5
Reactive oxygen species (ROS) are essential secondary messengers for rapid transmission of local and long-distance signalling in plants. Respiratory oxidase homologs (RBOHs) are the family of enzymes in plants that produce extracellular ROS, with RBOHD being the primary oxidase in Arabidopsis for ROS production following pathogen perception. Positive regulation of RBOHD by activating phosphorylation is well characterized, but it was unknown how RHOBD is inhibited pre- and post-pathogen recognition to maintain immune homeostasis. Lee et al. identified that PBS1-like kinase 13 (PBL13) phosphorylates conserved C-terminal residues on RBOHD to decrease its stability. They further identified the previously uncharacterized ubiquitin E3 ligase PIRE (PBL13 interacting RING domain E3 ligase), which polyubiquitinates RBOHD at residues near the PBL13-targetted phosphorylation sites, targeting it for endocytosis and vacuolar degradation. Mimicking phosphorylation at these sites directly promotes ubiquitination and protein turnover. These results showcase cross-talk between phosphorylation and ubiquitination as a mechanism to negatively regulate plant immunity. (Summary by Katy Dunning @plantmomkaty) Nature Comms 10.1038/s41467-020-15601-5
[altmetric doi=”10.1038/s41467-020-15601-5″ details=”right” float=”right”]