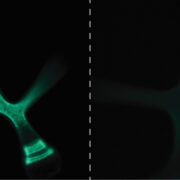
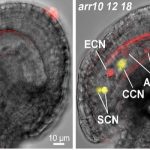
GPI lipid remodeling endows proteins with cell surface anchoring and affects cell wall biosynthesis
Zuopeng Xu1, Yihua Zhou2, Baocai Zhang2
1 College of Agriculture, Yangzhou University, Yangzhou, 225009, China
2 Institute of Genetics and Developmental Biology, The Innovative Academy of Seed Design, Chinese Academy of Sciences, Beijing 100101, China
Background: Glycosylphosphatidylinositol (GPI) anchoring is an important post-translational modification, which tethers proteins to the outer leaflet of the plasma membrane. Approximately 1% of plant proteins are thought to be modified with a GPI anchor and involved in many biological processes via facilitating signal perception, cell adhesion, transportation, and metabolism. Mature GPI moieties of eukaryotes usually contain a conserved glycan core structure and a variable lipid tail. The lipid portion is crucial for delivery of GPI-anchored proteins (GPI-APs) to specific lipid microdomains to carry out specialized functions. However, contrary to their known functional importance, plant GPI lipid composition and the biosynthetic pathway for GPI modification in plants remains to be explored.
Question: How are the lipid tails of GPI-APs formed in plants and what are their effects on cell wall formation?
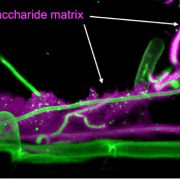
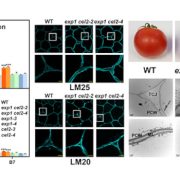
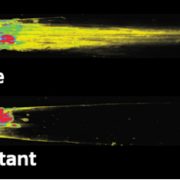
Findings: Through characterization of the rice (Oryza sativa) brittle culm 16 (bc16) mutant, we identified a membrane-bound O-acyltransferase required for GPI lipid modification. We found that BC16 is located in the endoplasmic reticulum and Golgi apparatus. BC16 acts as an acyltransferase in GPI lipid remodeling, based on the findings that BC16 rescued the growth defects of a yeast mutant deficient in a membrane-bound O-acyltransferase homolog, and that disruption of BC16 in rice causes an obvious reduction in GPI-AP lipids. Using BC1, a previously reported GPI-AP, as a reporter, we clarified that BC16-mediated lipid remodeling is required for targeting GPI-APs, including BC1, to plasma membrane lipid nanodomains. We further performed atomic force microscopy and nanoindentation analyses to reveal the impacts of BC16 on cell wall nanofiber organization and elastic moduli.
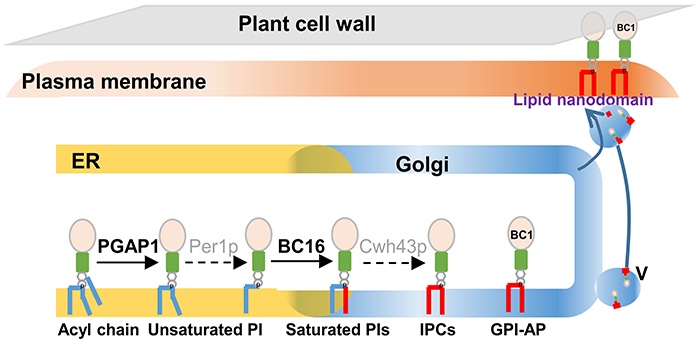
Next steps: We will identify more enzymes involved in plant GPI lipid maturation, which will shed light on mechanisms of GPI modification and offer a comprehensive understanding of plant GPI-AP functions in cell wall organization.
Reference:
Zuopeng Xu, Yihong Gao, Chengxu Gao, Jiasong Mei, Shaogan Wang, Jiaxin Ma, Hanlei Yang, Shaoxue Cao, Yan Wang, Fengxia Zhang, Xiangling Liu, Qiaoquan Liu, Yihua Zhou, Baocai Zhang (2022) Glycosylphosphatidylinositol anchor lipid remodeling directs proteins to the plasma membrane and governs cell wall mechanics. Plant Cell. https://doi.org/10.1093/plcell/koac257