Functional insights into the TPLATE complex: A key player in clathrin-mediated endocytosis in plant cells
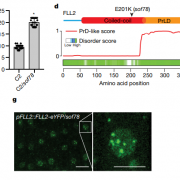
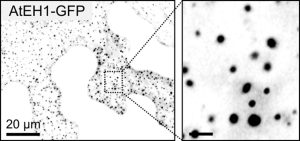 Transporting cargo within a cell may seem straightforward, but it is actually an intricate task. At the plasma membrane in plant cells, this complexity is navigated by a process known as clathrin-mediated endocytosis. Central to this process are two key players: adapter protein AP-2 and the TSET/TPLATE complex (TPC). As an evolutionarily ancient structure, the TPC has emerged as a vital component in this cellular machinery. Acting as an adapter module, it plays a crucial role in shaping the membrane during endocytosis and has been found to interact with ubiquitinated cargo. Structurally, it resembles both COPI and AP complexes; however, the TPC forms an octameric complex and is present during the earliest phases of endocytosis until vesicle scission and uncoating. In Dragwidge et al., the authors delved into the roles of two highly disordered TPC subunits, EH1 and EH2. They discovered that these subunits serve as scaffolds for biomolecular condensation of the complex, a process crucial for influencing endocytosis dynamics as well as recruiting other endocytic proteins. Specifically, the EH domain in EH1 and EH2 was identified as responsible for lipid binding, enabling interaction with the plasma membrane by binding anionic phospholipids. In contrast, the intrinsically disordered region1 (IDR1) affects condensate properties. The authors provide stunning high-resolution microscopic images reinforcing their findings. Their work emphasizes the significant role of condensation and phase separation in the assembly of clathrin-coated vesicles and the recruitment of other accessory proteins at the plasma membrane. (Summary by Ann-Kathrin Rößling @AK_Roessling) 10.1038/s41556-024-01354-6
Transporting cargo within a cell may seem straightforward, but it is actually an intricate task. At the plasma membrane in plant cells, this complexity is navigated by a process known as clathrin-mediated endocytosis. Central to this process are two key players: adapter protein AP-2 and the TSET/TPLATE complex (TPC). As an evolutionarily ancient structure, the TPC has emerged as a vital component in this cellular machinery. Acting as an adapter module, it plays a crucial role in shaping the membrane during endocytosis and has been found to interact with ubiquitinated cargo. Structurally, it resembles both COPI and AP complexes; however, the TPC forms an octameric complex and is present during the earliest phases of endocytosis until vesicle scission and uncoating. In Dragwidge et al., the authors delved into the roles of two highly disordered TPC subunits, EH1 and EH2. They discovered that these subunits serve as scaffolds for biomolecular condensation of the complex, a process crucial for influencing endocytosis dynamics as well as recruiting other endocytic proteins. Specifically, the EH domain in EH1 and EH2 was identified as responsible for lipid binding, enabling interaction with the plasma membrane by binding anionic phospholipids. In contrast, the intrinsically disordered region1 (IDR1) affects condensate properties. The authors provide stunning high-resolution microscopic images reinforcing their findings. Their work emphasizes the significant role of condensation and phase separation in the assembly of clathrin-coated vesicles and the recruitment of other accessory proteins at the plasma membrane. (Summary by Ann-Kathrin Rößling @AK_Roessling) 10.1038/s41556-024-01354-6