Cryo-EM structure of OSCA1.2 sheds light on the mechanical basis of membrane hyperosmolality gating (PNAS) ($)
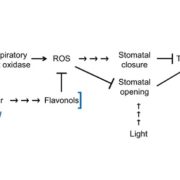
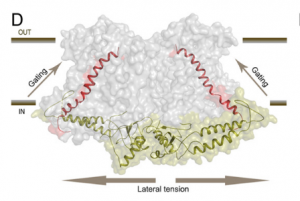 Osmotic stress in plants elicits many responses, one of which is increased accumulation of Ca2+ in the cytosol. Genes involved in this response have been identified, yet the mechanism behind the Ca2+ transport remains unknown. Maity et al. investigated the structure and function of the osmolality-sensitive channel OSCA1.2, a candidate for hyperosmolarity-induced Ca2+ transport, using cryo-electron microscopy along with reconstitution of the protein in droplet-interfaced bilayers. They demonstrated that OSCA1.2 is dimeric, with each monomer containing 11 transmembrane domains (TMDs), as well as a large cytosolic loop between TM2 and TM3 that mediates the dimeric interaction. The predicted transport pathway contains 5 amino acids contributed from TM5 and TM6. Based on computer simulations, these may interact with the cytosolic loop to sense conformational changes that can be translated to transport activity under hyperosmotic scenarios. However, a Ca2+ fluorescent dye and two-electrode voltage clamping with Xenopus oocytes didn’t provide evidence of osmotic stress-induced Ca2+ flux. Therefore, substrate identity and the gating mechanism of OSCA1.2 remain questions for future research. (Summary by Nathan Scinto-Madonich) PNAS 10.1073/pnas.1900774116 .
Osmotic stress in plants elicits many responses, one of which is increased accumulation of Ca2+ in the cytosol. Genes involved in this response have been identified, yet the mechanism behind the Ca2+ transport remains unknown. Maity et al. investigated the structure and function of the osmolality-sensitive channel OSCA1.2, a candidate for hyperosmolarity-induced Ca2+ transport, using cryo-electron microscopy along with reconstitution of the protein in droplet-interfaced bilayers. They demonstrated that OSCA1.2 is dimeric, with each monomer containing 11 transmembrane domains (TMDs), as well as a large cytosolic loop between TM2 and TM3 that mediates the dimeric interaction. The predicted transport pathway contains 5 amino acids contributed from TM5 and TM6. Based on computer simulations, these may interact with the cytosolic loop to sense conformational changes that can be translated to transport activity under hyperosmotic scenarios. However, a Ca2+ fluorescent dye and two-electrode voltage clamping with Xenopus oocytes didn’t provide evidence of osmotic stress-induced Ca2+ flux. Therefore, substrate identity and the gating mechanism of OSCA1.2 remain questions for future research. (Summary by Nathan Scinto-Madonich) PNAS 10.1073/pnas.1900774116 .