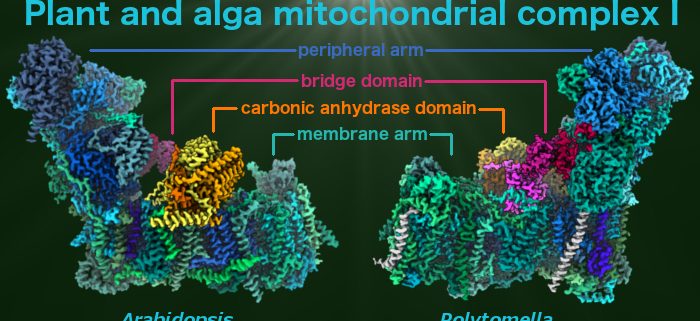
Atomic structure of mitochondrial complex I from plants and algae
Klusch et al. discover an unusual bridge domain that might participate in regulating the activity of mitochondrial complex I in plants and algae.
By Niklas Klusch1, Jennifer Senkler2, Özkan Yildiz1, Werner Kühlbrandt1 and Hans-Peter Braun2
1 Department of Structural Biology, Max-Planck-Institute of Biophysics, Max-von-Laue-Straße 3, 60438 Frankfurt, Germany
2 Institut für Pflanzengenetik, Leibniz Universität Hannover, Herrenhäuser Str. 2, 30419 Hannover, Germany
Background: Photosynthesis not only provides the energy needed for carbon, nitrogen, and sulfur assimilation, but it also mediates redox regulation of plant metabolism, which is very different in the daytime and at night. This regulation also affects cellular respiration, which takes place in the mitochondria. Our paper reports the atomic structure of the plant and algal NADH dehydrogenase complex, the first enzyme complex of the mitochondrial respiratory chain (also simply called complex I). Complex I from plants and algae has a conspicuous extra domain of three carbonic anhydrase enzymes that has been suggested to link mitochondrial metabolism to carbon assimilation in the chloroplasts. Redox regulation may be needed to adapt complex I to its different roles during the day-night cycle. The structural prerequisites for this anticipated regulation are not known.
Question: What are the special features of mitochondrial complex I in algae and plants? Is the carbonic anhydrase domain of plant complex I a structural module, a functional domain, or both? How does plant complex I work and how is it regulated?
Findings: We used electron cryo-microscopy (cryo-EM) to determine the atomic structure of mitochondrial complex I from the model plant Arabidopsis thaliana and from Polytomella, a non-photosynthetic green alga, at around 2.9 Ångstrom resolution. Complex I consists of two elongated domains called the membrane arm and the peripheral arm, which together form a conserved L-shape. In Arabidopsis, the carbonic anhydrase domain attached to the membrane arm includes a Zn2+ ion in one of its three active sites, and we therefore think it is enzymatically active. Furthermore, we discovered an unusual ferredoxin in a protein bridge that links the carbonic anhydrase domain to the peripheral arm of complex I. The bridge seems to set the angle between the two complex I arms, which in mammals is relevant for activity. We propose that the ferredoxin allows for redox-mediated regulation of complex I in plants.
Next steps: The new ferredoxin we discovered needs to be analyzed biochemically, physiologically, and genetically to test its proposed function in redox-mediated regulation of plant complex I.
Niklas Klusch, Jennifer Senkler, Özkan Yildiz, Werner Kühlbrandt, Hans-Peter Braun (2021). A ferredoxin bridge connects the two arms of plant mitochondrial complex I. Plant Cell https://doi.org/10.1093/plcell/koab092