A regulatory module for survival on low potassium (Nature Plants)
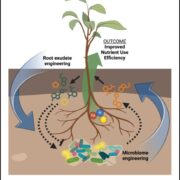
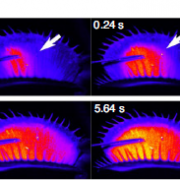
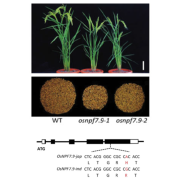
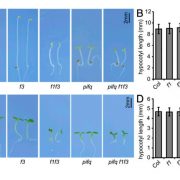
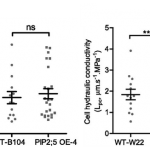
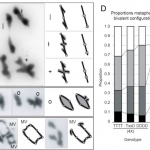
 Potassium ions (K+) play a variety of important roles in plant physiology and are maintained at a high concentration in the cytosol relative to the available K+ in the environment. Potassium may accumulate to an even higher concentration in the vacuole, where it helps to regulate turgor pressure. During periods of potassium scarcity, the plant can increase the cytoplasmic K+ concentration by mobilizing these vacuolar stores. In this paper, Tang et al. describe a pathway that contributes to potassium homeostasis by increasing efflux from the vacuole during low-K+ stress. They show that a specific set of calcium-binding sensor proteins (CBL2 and CBL3) are important for successful germination in low-K+ media, presumably due to increased reliance on internal stores. A similar phenotype resulted from deletion of CBL-interacting protein kinases, especially CIPK9. Current recordings from isolated vacuoles demonstrated that CBLs and CIPKs are indeed important for potassium flux. But how does the CBL-CIPK module change the conductance of the tonoplast towards potassium? One candidate third component is the tonoplast-localized channel TPK1. By co-expressing all three proteins in protoplast vacuoles, they show that the CBL3-CIPK9-TPK1 complex is sufficient to boost potassium efflux, and that this effect is entirely dependent on the kinase activity of CIPK9. Altogether, this study adds new molecular detail to how plants regulate internal potassium levels in response to a changing environment. (Summary by Frej Tulin @FrejTulin) Nature Plants 10.1038/s41477-020-0621-7
Potassium ions (K+) play a variety of important roles in plant physiology and are maintained at a high concentration in the cytosol relative to the available K+ in the environment. Potassium may accumulate to an even higher concentration in the vacuole, where it helps to regulate turgor pressure. During periods of potassium scarcity, the plant can increase the cytoplasmic K+ concentration by mobilizing these vacuolar stores. In this paper, Tang et al. describe a pathway that contributes to potassium homeostasis by increasing efflux from the vacuole during low-K+ stress. They show that a specific set of calcium-binding sensor proteins (CBL2 and CBL3) are important for successful germination in low-K+ media, presumably due to increased reliance on internal stores. A similar phenotype resulted from deletion of CBL-interacting protein kinases, especially CIPK9. Current recordings from isolated vacuoles demonstrated that CBLs and CIPKs are indeed important for potassium flux. But how does the CBL-CIPK module change the conductance of the tonoplast towards potassium? One candidate third component is the tonoplast-localized channel TPK1. By co-expressing all three proteins in protoplast vacuoles, they show that the CBL3-CIPK9-TPK1 complex is sufficient to boost potassium efflux, and that this effect is entirely dependent on the kinase activity of CIPK9. Altogether, this study adds new molecular detail to how plants regulate internal potassium levels in response to a changing environment. (Summary by Frej Tulin @FrejTulin) Nature Plants 10.1038/s41477-020-0621-7