A plant kinase exploited by a bacterial effector (bioRxiv)
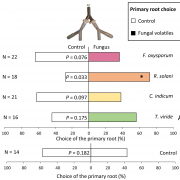
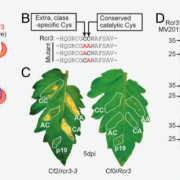
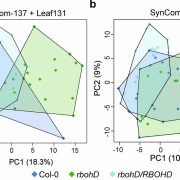
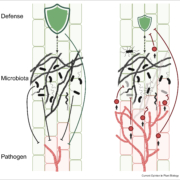
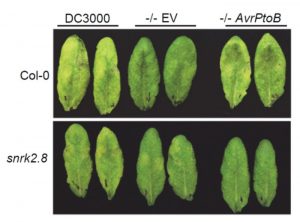 Pathogens have evolved a suite of effector proteins that are secreted into plants and aid successful colonization. AvrPtoB is a well-conserved effector of pathogenic Pseudomonas syringae strains that targets multiple plant defense-related proteins and is required for pathogen virulence and bacterial growth in plants. Phosphorylation of AvrPtoB in plants is important for its virulence functions, but the mechanism by which the effector is phosphorylated is unknown. Lei et al. demonstrated that the plant kinase SnRK2.8, a member of a widely conserved plant kinase family, interacts with AvrPtoB and phosphorylates this effector protein in Arabidopsis thaliana. The virulence functions of AvrPtoB were abolished in the absence of SnRK2.8 or when the phosphorylation sites are blocked. SnRK2.8 was proposed to phosphorylate the key plant defense regulator NPR1 to mediate plant immunity. This mechanism appears to be exploited by AvrPtoB, which, upon phosphorylation, degrades NPR1. This study identifies the first plant kinase exploited by a bacterial pathogen to phosphorylate and activate an effector protein illuminating an evolutionary arms race between plants and pathogens. (Summary by Tatsuya Nobori) bioRxiv 10.1101/2020.03.21.001826v1
Pathogens have evolved a suite of effector proteins that are secreted into plants and aid successful colonization. AvrPtoB is a well-conserved effector of pathogenic Pseudomonas syringae strains that targets multiple plant defense-related proteins and is required for pathogen virulence and bacterial growth in plants. Phosphorylation of AvrPtoB in plants is important for its virulence functions, but the mechanism by which the effector is phosphorylated is unknown. Lei et al. demonstrated that the plant kinase SnRK2.8, a member of a widely conserved plant kinase family, interacts with AvrPtoB and phosphorylates this effector protein in Arabidopsis thaliana. The virulence functions of AvrPtoB were abolished in the absence of SnRK2.8 or when the phosphorylation sites are blocked. SnRK2.8 was proposed to phosphorylate the key plant defense regulator NPR1 to mediate plant immunity. This mechanism appears to be exploited by AvrPtoB, which, upon phosphorylation, degrades NPR1. This study identifies the first plant kinase exploited by a bacterial pathogen to phosphorylate and activate an effector protein illuminating an evolutionary arms race between plants and pathogens. (Summary by Tatsuya Nobori) bioRxiv 10.1101/2020.03.21.001826v1
[altmetric doi=”10.1101/2020.03.21.001826v1″ details=”right” float=”right”]