A Lectin Receptor Kinase as a Potential Sensor for Extracellular Nicotinamide Adenine Dinucleotide in Arabidopsis thaliana (OA)
 eLife Besides its role as an enzyme cofactor, NAD is also a plant signaling molecule. In particular, it leaks from wounded areas and activates defense pathways in the neighboring tissue, an effect that can be mimicked by application of extracellular NAD.
eLife Besides its role as an enzyme cofactor, NAD is also a plant signaling molecule. In particular, it leaks from wounded areas and activates defense pathways in the neighboring tissue, an effect that can be mimicked by application of extracellular NAD.
The presence of a sensing mechanism for extracellular NAD levels was therefore suggested. To identify potential NAD membrane receptors, a microarray analysis of exogenous NAD-induced genes was performed and LecRK-I.8 was identified as a good candidate. The authors demonstrated that LecRK-I.8 is localized at the plasma membrane and that its intracellular domain exhibits kinase activity.
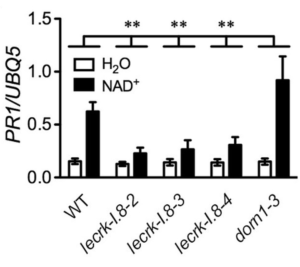
Although the NAD binding parameters are not fully in line with the extracellular NAD levels able to trigger a defense response, it was shown that LecRK-I.8 can bind NAD but not other related molecules such as NADP. Furthermore, mutations of the putative NAD binding site block NAD activation of defense genes but not NADP+-, flg22-, and SA-induced defense responses, thus corroborating its role as a specific NAD receptor.
This work establishes NAD signaling as part of the defense response of Arabidopsis. Further work is required to confirm these findings in other plant species and identify other components of the signaling pathway. (Summary by Elisa Dell’Aglio) eLife 10.7554/eLife.25474