Understanding Evening Complex activity (PNAS)
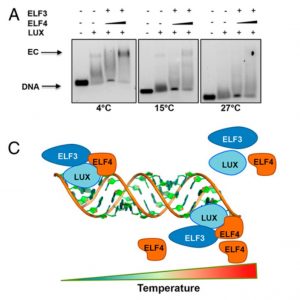 Processes like photosynthesis and growth follow a well-defined rhythm driven by a 24-h internal clock that is controlled by three principal interacting transcription–translation feedback loops: the morning, central, and evening loops. The Evening Complex (EC), which is active at the end of the day, participates in the transcriptional repression of the morning loop genes. In a recent study, Silva and colleagues provided an in vitro model for Evening Complex (EC) activity, including binding affinity, specificity, and complex formation. EC is composed of LUX ARRHYTHMO (LUX), EARLY FLOWERING 3 (ELF3), and ELF4. Since LUX is the only protein in the complex with a DNA-binding capacity, the authors used protein binding microarrays to determine its affinity and specificity. Structural analysis of the LUX-DNA complex allowed the identification of critical residues involved in DNA-binding. With this information, authors generated a version of LUX with reduced binding affinity, LUXR146A, which has an in vivo effect. To determine the role of ELF3 and ELF4 in the EC formation and function, authors reconstituted different sub-complexes in vitro and analyzed their DNA-binding features. LUX-ELF3 has low DNA binding capacity, and the addition of EFL4 results in an increase in the high-affinity DNA-binding. The DNA-binding capacity of LUX alone is not temperature-dependent, but with increased temperature the binding of the whole complex is attenuated. In this scenario, ELF4 confirms its prominent role in the EC binding stabilization, since high concentrations of ELF4 (~20-fold excess) can restore DNA-binding at high temperatures. (Summary by Humberto Herrera-Ubaldo) Proc. Natl. Acad. Sci. USA
Processes like photosynthesis and growth follow a well-defined rhythm driven by a 24-h internal clock that is controlled by three principal interacting transcription–translation feedback loops: the morning, central, and evening loops. The Evening Complex (EC), which is active at the end of the day, participates in the transcriptional repression of the morning loop genes. In a recent study, Silva and colleagues provided an in vitro model for Evening Complex (EC) activity, including binding affinity, specificity, and complex formation. EC is composed of LUX ARRHYTHMO (LUX), EARLY FLOWERING 3 (ELF3), and ELF4. Since LUX is the only protein in the complex with a DNA-binding capacity, the authors used protein binding microarrays to determine its affinity and specificity. Structural analysis of the LUX-DNA complex allowed the identification of critical residues involved in DNA-binding. With this information, authors generated a version of LUX with reduced binding affinity, LUXR146A, which has an in vivo effect. To determine the role of ELF3 and ELF4 in the EC formation and function, authors reconstituted different sub-complexes in vitro and analyzed their DNA-binding features. LUX-ELF3 has low DNA binding capacity, and the addition of EFL4 results in an increase in the high-affinity DNA-binding. The DNA-binding capacity of LUX alone is not temperature-dependent, but with increased temperature the binding of the whole complex is attenuated. In this scenario, ELF4 confirms its prominent role in the EC binding stabilization, since high concentrations of ELF4 (~20-fold excess) can restore DNA-binding at high temperatures. (Summary by Humberto Herrera-Ubaldo) Proc. Natl. Acad. Sci. USA
[altmetric doi=”10.1073/pnas.1920972117″ details=”right” float=”right”]