OsLESV cooperates with FLO6 to modulate starch biosynthesis and endosperm development
Yan, Zhang, Wang et al. identify OsLESV–FLO6 as a non-enzymatic molecular module responsible for starch biosynthesis and endosperm development.
https://doi.org/10.1093/plcell/koae006
By Haigang Yan, Wenwei Zhang and Jianmin Wan
State Key Laboratory of Crop Genetics & Germplasm Enhancement and Utilization, Nanjing Agricultural University, Nanjing 210095, China
Background: Starch accounts for up to 75% of grain weight, and greatly affects cereal crop yield and quality. Significant advances have been made in the functional characterization of starch metabolism enzymes in cereal crops. In addition to starch metabolism enzymes, some non-enzymatic players with starch-binding domains play crucial roles in starch biosynthesis. ISOAMYLASE 1 (ISA1), a type of debranching enzyme unable to bind starch, functions in starch biosynthesis through removing superfluous branch points. However, how ISA1 is targeted to starch granules remains largely unknown.
Question: Comparable phenotypic defects between floury endosperm 9 (flo9) and isa1 prompted us to propose that LIKE EARLY STARVATION1 (OsLESV), the target protein responsible for the flo9 phenotype, might regulate rice (Oryza sativa) starch biosynthesis by associating with ISA1. Therefore, we asked whether OsLESV is a non-enzymatic player that facilitates ISA1 targeting to starch.
Findings: We determined that the rice flo9 mutant is defective in starch biosynthesis and endosperm development, similar to the reported isa1 mutants. FLO9 encodes an amyloplast-localized protein homologous to Arabidopsis (Arabidopsis thaliana) LESV. OsLESV is required for compound starch granule initiation in the endosperm. OsLESV directly binds to starch by its C-terminal Trp-rich region. We demonstrated that OsLESV interacts with the reported non-enzymatic player FLO6, and loss-of-function of either gene impairs ISA1 targeting to starch granules. Genetically, OsLESV acts synergistically with FLO6 to modulate starch biosynthesis and endosperm development. Our findings establish a molecular link between non-enzymatic players and ISA1 in rice starch biosynthesis and endosperm development.
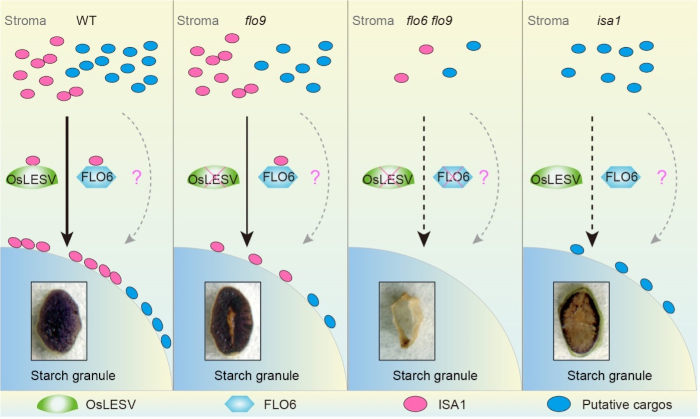
Next steps: In addition to facilitating ISA1 localization on starch granules, OsLESV likely exerts other functions in starch biosynthesis and endosperm development. Therefore, it is important to identify other unknown cargos to fully understand the biological significance of OsLESV in regulating starch metabolism in future studies.
Reference:
Haigang Yan, Wenwei Zhang, Yihua Wang, Jie Jin, Hancong Xu, Yushuang Fu, Zhuangzhuang Shan, Xin Wang, Xuan Teng, Xin Li, Yongxiang Wang, Xiaoqing Hu, Wenxiang Zhang, Changyuan Zhu, Xiao Zhang, Yu Zhang, Rongqi Wang, Jie Zhang, Yue Cai, Xiaoman You, Jie Chen, Xinyuan Ge, Liang Wang, Jiahuan Xu, Ling Jiang, Shijia Liu, Cailin Lei, Xin Zhang, Haiyang Wang, Yulong Ren, and Jianmin Wan. (2023). Rice LIKE EARLY STARVATION1 cooperates with FLOURY ENDOSPERM 6 to modulate starch biosynthesis and endosperm development. https://doi.org/10.1093/plcell/koae006
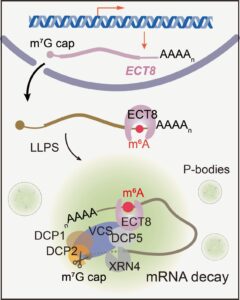 Question: We wanted to know how ECT8 interacts with this tagged RNA and what happens to the RNA afterwards, especially when the plant is dealing with too much salt.
Question: We wanted to know how ECT8 interacts with this tagged RNA and what happens to the RNA afterwards, especially when the plant is dealing with too much salt.

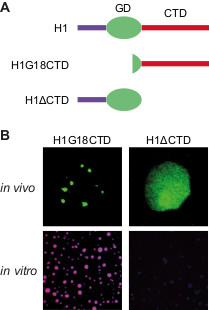 Findings: Our study reveals that H1 condenses heterochromatin in Arabidopsis through a phase separation mechanism. This mechanism refers to the self-aggregating property of macromolecules, leading to the formation of distinct phases. A simple example of this phenomenon is the spontaneous separation of oil and water after mixing. We find that the C-terminal domain (CTD) of H1 mediates chromatin phase separation in vitro and in vivo. Without the CTD, H1 no longer drives the formation of heterochromatin foci, or performs normal functions such as regulating nucleosomal repeat length and DNA methylation. Thus, CTD-endorsed phase separation is the primary mechanism by which histone H1 promotes heterochromatin condensation or achieves its function in heterochromatin.
Findings: Our study reveals that H1 condenses heterochromatin in Arabidopsis through a phase separation mechanism. This mechanism refers to the self-aggregating property of macromolecules, leading to the formation of distinct phases. A simple example of this phenomenon is the spontaneous separation of oil and water after mixing. We find that the C-terminal domain (CTD) of H1 mediates chromatin phase separation in vitro and in vivo. Without the CTD, H1 no longer drives the formation of heterochromatin foci, or performs normal functions such as regulating nucleosomal repeat length and DNA methylation. Thus, CTD-endorsed phase separation is the primary mechanism by which histone H1 promotes heterochromatin condensation or achieves its function in heterochromatin.