Linker histone H1 drives heterochromatin condensation
He et al. explore how histone H1 drives the formation of heterochromatin foci.
https://doi.org/10.1093/plcell/koae034
Xiaoqi Feng, Institute of Science and Technology Austria
Background: To fit into the nucleus, DNA is densely packaged into structures known as nucleosomes. These nucleosomes consist of core histone proteins around which DNA is wrapped, along with linker histone protein H1, which binds to the DNA between nucleosomes. Parts of the DNA containing repetitive sequences that do not code but can jump around the genome and disrupt genes are further condensed into structures known as heterochromatin foci. These foci appear as visible, dense structures under a microscope, and maintain the silencing of repetitive sequences. H1 proteins are required for the formation of heterochromatin foci and the silencing of repetitive sequences in animals and plants.
Questions: How does H1 induce the formation of heterochromatin foci? Is the formation of heterochromatin foci essential for H1’s functions in the chromatin?
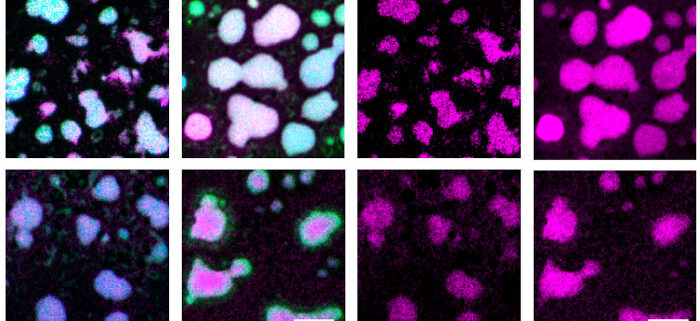
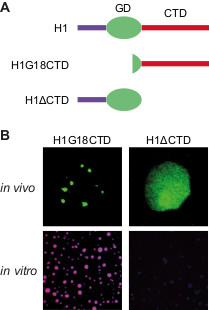 Findings: Our study reveals that H1 condenses heterochromatin in Arabidopsis through a phase separation mechanism. This mechanism refers to the self-aggregating property of macromolecules, leading to the formation of distinct phases. A simple example of this phenomenon is the spontaneous separation of oil and water after mixing. We find that the C-terminal domain (CTD) of H1 mediates chromatin phase separation in vitro and in vivo. Without the CTD, H1 no longer drives the formation of heterochromatin foci, or performs normal functions such as regulating nucleosomal repeat length and DNA methylation. Thus, CTD-endorsed phase separation is the primary mechanism by which histone H1 promotes heterochromatin condensation or achieves its function in heterochromatin.
Findings: Our study reveals that H1 condenses heterochromatin in Arabidopsis through a phase separation mechanism. This mechanism refers to the self-aggregating property of macromolecules, leading to the formation of distinct phases. A simple example of this phenomenon is the spontaneous separation of oil and water after mixing. We find that the C-terminal domain (CTD) of H1 mediates chromatin phase separation in vitro and in vivo. Without the CTD, H1 no longer drives the formation of heterochromatin foci, or performs normal functions such as regulating nucleosomal repeat length and DNA methylation. Thus, CTD-endorsed phase separation is the primary mechanism by which histone H1 promotes heterochromatin condensation or achieves its function in heterochromatin.
Next steps: Our data also suggest that bacterial H1-like proteins, which resemble the CTD of eukaryotic H1 proteins and have been implicated in DNA condensation, can mediate phase separation. Therefore, we propose that phase separation mediated by H1 and H1-like proteins represents an ancient mechanism for compacting chromatin and DNA. Rigorous tests are required to substantiate this idea.
Reference:
Shengbo He, Yiming Yu, Liang Wang, Guohong Li, Pilong Li & Xiaoqi Feng. (2024). Linker histone H1 drives heterochromatin condensation via phase separation in Arabidopsis https://doi.org/10.1093/plcell/koae034