A biosensor for ER redox dynamics highlights ER oxidoreductins
Ugalde et al. report that dynamic monitoring of the ER luminal glutathione redox potential highlights the role of ER oxidoreductins in defining redox conditions and the interplay between different redox inputs during hypoxia and reductive stress. Plant Cell https://doi.org/10.1093/plcell/koac202
Background: Most secreted proteins contain disulfide bridges that are essential for their structure and function. Those disulfides are introduced into the nascent polypeptide through the oxidation of cysteines in the endoplasmic reticulum (ER) lumen. Oxidative protein folding requires molecular oxygen (O2) as ultimate electron acceptor. The final electron transfer is catalyzed by thiol oxidases called ER oxidoreductins (EROs).
Question: What is the role of EROs in maintaining ER redox homeostasis at steady state and when oxygen supply is limiting?
Finding: Arabidopsis thaliana contains two ERO genes. An ero1 ero2 double mutant generated by combining a null allele for ERO1 with a knockdown allele for ERO2 showed enhanced sensitivity towards thiol-based reductive challenge and hypoxia. By monitoring the glutathione redox potential EGSH in the ER lumen using the redox biosensor variant roGFP2iL we measured -241 mV in the wild type, which is a less oxidizing value than previously thought. A good match between the midpoint potential of the biosensor variant and the physiological EGSH in the ER lumen enabled dynamic measurements indicating ERO activity in vivo. Diminished ERO activity in ero1 ero2 caused a reductive shift to -253 mV and delayed recovery after reductive challenge. The dynamics of luminal EGSH under hypoxia in ero1 ero2 differed from the response obtained in wild-type plants, indicating that ERO activity plays a key role in luminal redox homeostasis.
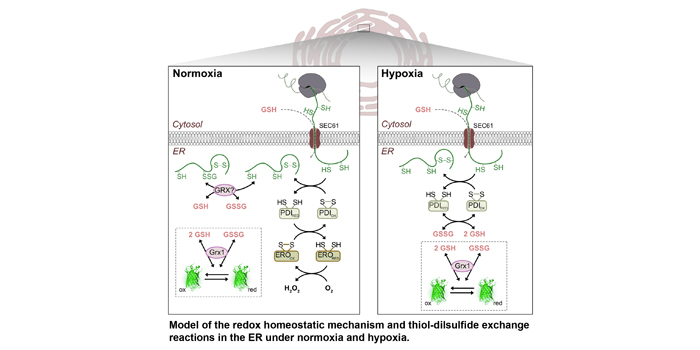 Next steps: Monitoring luminal EGSH represents a platform for evaluating ER redox dynamics and allows assessing other candidates for their potential contribution to oxidative protein folding and maintaining luminal redox homeostasis. Future research may focus on the integration of ER redox homeostasis and phytohormone signaling especially under stress situations or during developmental phases associated with hypoxic conditions.
Next steps: Monitoring luminal EGSH represents a platform for evaluating ER redox dynamics and allows assessing other candidates for their potential contribution to oxidative protein folding and maintaining luminal redox homeostasis. Future research may focus on the integration of ER redox homeostasis and phytohormone signaling especially under stress situations or during developmental phases associated with hypoxic conditions.
José Manuel Ugalde, Isabel Aller, Lika Kudrjasova, Romy R. Schmidt, Michelle Schlößer, Maria Homagk, Philippe Fuchs, Sophie Lichtenauer, Markus Schwarzländer, Stefanie J. Müller-Schüssele, Andreas J. Meyer. (2022). Endoplasmic reticulum oxidoreductin provides resilience against reductive stress and hypoxic conditions by mediating luminal redox dynamics. Plant Cell. https://doi.org/10.1093/plcell/koac202