Alternative complex III: an ancient counterpart of the bc1/b6f complex
Xin et al. determined the cryo-EM structure of HQNO-bound Alternative Complex III from the anoxygenic phototrophic bacterium Chloroflexus aurantiacus. The Plant Cell (2024)
https://doi.org/10.1093/plcell/koae029
By Xiaoling Xu at Hangzhou Normal University, Hangzhou, China
Background: Photosynthesis and cellular respiration are complementary biological processes that play essential roles in sustaining the balance of oxygen and carbon dioxide cycling on Earth. In both processes, the cytochrome bc1/b6f complex, also known as Complex III, couples the electron transport chain with transmembrane proton translocation to generate proton motive force (PMF), which drives ATP synthesis and other energy-consuming activities. Studying similar complexes in other organisms can shed light on how these Complex III functions. Indeed, many bacterial species have an alternative complex III (ACIII), which differs in composition and structurally from the bc1/b6f complex, but functionally replaces the bc1/b6f complex. However, the true compositions and architectures of ACIIIs remain unclear, as do their structural and functional relevance in mediating the redox-coupled transmembrane proton translocation.
Question: Structural studies of ACIIIs have revealed a highly conserved spatial organization of six core subunits (ActA–F), a potential quinol-binding site, and an electron transfer wire composed of [3Fe-4S] and six c-type hemes (five in ActA and one in ActE) leading to the periplasmic electron acceptor. However, the molecular mechanisms underlying quinol oxidation and electron transfer are unclear, due to lack of intact complex conformations with bound (mena)quinols or analogs in any of the reported structures.
Findings: Here we determined the cryo-EM structures of photosynthetic ACIII isolated from the filamentous anoxygenic phototrophic bacterium Chloroflexus aurantiacus (CaACIIIp), in apo-form and in complexed form bound to a menadiol analog 2-heptyl-4-hydroxyquinoline-N-oxide (HQNO). We revealed conformations of two subunits, ActG and I, which contributed to complex stability. Through a combination of electron paramagnetic resonance, spectroelectrochemical, enzymatic, comparative structural analyses and molecular dynamics simulations, we characterized the (mena)quinol binding pocket, the pathway by which electrons are transferred through the complex to acceptor auracyanin, and a unique insertion loop in ActE that functions in determining the binding specificity of CaACIIIp for downstream electron acceptors.
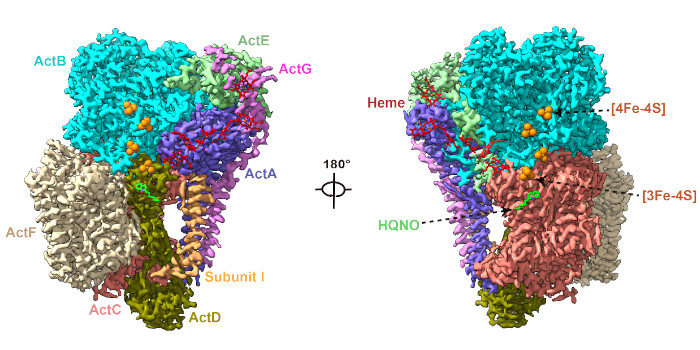
Chloroflexus aurantiacus alternative complex III (CaACIIIp) mediated menaquinol oxidation and electron transfer to auracyanin-A and -B.
Next steps: Previous studies proposed that ACIII could also actively pump additional protons from cytoplasm into periplasm. To date, we lack experimental data on the proton translocation activity for any ACIII, leaving the redox-coupled proton translocation mechanism unclear. Future studies should focus on experimental validation of this activity.
Reference:
Jiyu Xin, Zhenzhen Min, Lu Yu, Xinyi Yuan, Aokun Liu, Wenping Wu, Xin Zhang, Huimin He, Jingyi Wu, Yueyong Xin, Robert E Blankenship, Changlin Tian, Xiaoling Xu (2024) Cryo-EM structure of HQNO-bound Alternative Complex III from the anoxygenic phototrophic bacterium Chloroflexus aurantiacus https://doi.org/10.1093/plcell/koae029